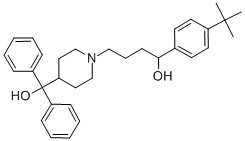
Terfenadine
- CAS No.
- 50679-08-8
- Chemical Name:
- Terfenadine
- Synonyms
- seldane;Terfenadin;Cyater;Terdin;terfen;Terfex;Aldaban;mdl9918;rmi9918;Teldane
- CBNumber:
- CB7695393
- Molecular Formula:
- C32H41NO2
- Molecular Weight:
- 471.67
- MDL Number:
- MFCD00079622
- MOL File:
- 50679-08-8.mol
| Melting point | 145-152 °C |
|---|---|
| Boiling point | 572.76°C (rough estimate) |
| Density | 1.0488 (rough estimate) |
| refractive index | 1.6310 (estimate) |
| storage temp. | 2-8°C |
| solubility | chloroform: soluble250 mg plus 5 ml of solvent, clear to very slightly hazy, colorless to faintly yellow |
| pka | pKa 9.21(H2O t = 25 I = 0.025) (Uncertain) |
| color | White to Off-White |
| Water Solubility | 0.001 g/100 mL (30 ºC) |
| CAS DataBase Reference | 50679-08-8(CAS DataBase Reference) |
| FDA UNII | 7BA5G9Y06Q |
| ATC code | R06AX12 |
| NIST Chemistry Reference | Seldane(50679-08-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H413 |
| Precautionary statements | P273-P501 |
| Safety Statements | 24/25 |
| WGK Germany | 2 |
| RTECS | TM4969000 |
| HS Code | 29333999 |
| Toxicity | LD50 orally in mice: >2000 mg/kg (Carr, Meyer) |
Terfenadine price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | T9652 | Terfenadine | 50679-08-8 | 5g | $110 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP650 | Terfenadine British Pharmacopoeia (BP) Reference Standard | 50679-08-8 | 100MG | $247 | 2024-03-01 | Buy |
| Alfa Aesar | J61936 | Terfenadine | 50679-08-8 | 100mg | $286 | 2023-06-20 | Buy |
| Cayman Chemical | 20305 | Terfenadine ≥98% | 50679-08-8 | 500mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 20305 | Terfenadine ≥98% | 50679-08-8 | 1g | $55 | 2024-03-01 | Buy |
Terfenadine Chemical Properties,Uses,Production
Chemical Properties
White Solid
Originator
Histafen,Berk
Uses
Terfenadine has been used to study the role of histamine in itch related to proteinase-activated receptors (PARs) in mice. Terfenadine has also been used to block histamine receptor type 1 to study the pathogenesis of 2,4-dinitrobenzene sulfonic acid (DNBS)-induced ulcerative colitis in rats.
Uses
It is used for relieving symptoms associated with seasonal allergic rhinitis and conjunctivitis, for angioneurotic edema and allergic skin reaction, and also as an ingredient of complex therapy for bronchial asthma. Synonyms of this drug are seldane, hystadin, trexil, and others.
Uses
H1 antihistamine
Uses
Nonsedating-type histamine H1-receptor antagonist. Antihistaminic
Definition
ChEBI: Terfenadine is a diarylmethane.
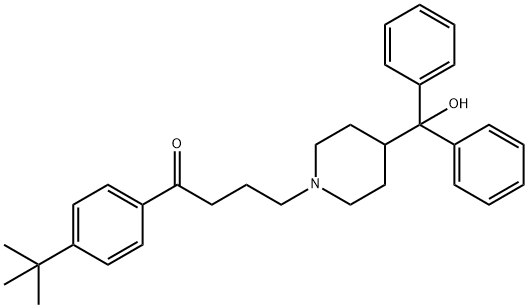
Manufacturing Process
A mixture of 107 g (0.4 mole) of α,α-diphenyl-4-piperidinemethanol, 105 g
(0.44 mole) of 4'-tert-butyl-4-chlorobutyrophenone, 70 g (0.7 mole) of
potassium bicarbonate, and a small amount of potassium iodide in 600 ml of
toluene was refluxed and stirred for 2.5 days then filtered. The filtrate was
treated with charcoal, filtered through celite then treated with ethereal HCl.
The resulting solid was recrystallized from methanol and isopropyl alcohol to
give the 4'-tert-butyl-4-[4-(α-hydroxy-α-phenylbenzyl)piperidino]-
butyrophenone hydrochloride, melting point 234°-235°C.
To a mixture of 4.2 g (0.0083 mole) of 4'-tert-butyl-4-[4-(α-hydroxy-α-
phenylbenzyl)piperidino]-butyrophenone hydrochloride and 0.54 g (0.01 mole)
of sodium methoxide in 25 ml of methanol is added 2.16 g (0.04 mole) of
potassium borohydride. The reaction mixture is stirred overnight, diluted with
water and the methanol removed under reduced pressure. The remaining
material is extracted with chloroform, washed with water, dried over
magnesium sulfate and filtered. The filtrate is concentrated, and the residue is
recrystallized from acetone-water to give 4-[α-(p-tert-butylphenyl)-α-
hydroxybenzyl]-α-phenyl-1-piperidinebutanol, melting point 161°-163°C.
brand name
Antifen;Fenadin.
Therapeutic Function
Antihistaminic, Bronchodilator
World Health Organization (WHO)
The first clinically interesting histamine H-receptor1 antagonists were introduced in the late 1940s and early 1950s. Several H-antihistaminics have a similar cardiac effect to that seen with astemizole1 and terfenadine. Serious cardiovascular adverse reactions have been reported when used concomitantly with imidazole antifungals and macrolide antibiotics. See also under astemizole.
Biological Activity
Histamine H 1 receptor antagonist. Also blocks hERG and K ATP channels (IC 50 values are 204 nM and 1.2 μ M respectively). Inhibits the delayed rectifier K + current (I Kr ) in guinea pig ventricular myocytes (IC 50 = 50 nM). Activity prolongs QT and induces Torsades de pointes (TdP); cardiotoxic in vivo .
Biochem/physiol Actions
Non-sedating second generation H1 histamine receptor antagonist. Mainly metabolized by Cyp3A4, 5, 7. Inhibits CYP2C8.
Pharmacology
Terfenadine not only differs from the other antihistamine drugs in its chemical structure, but also in that its action begins within 1–2 h and last approximately 12 h, reaching its peak of action in 3–4 h.
Synthesis
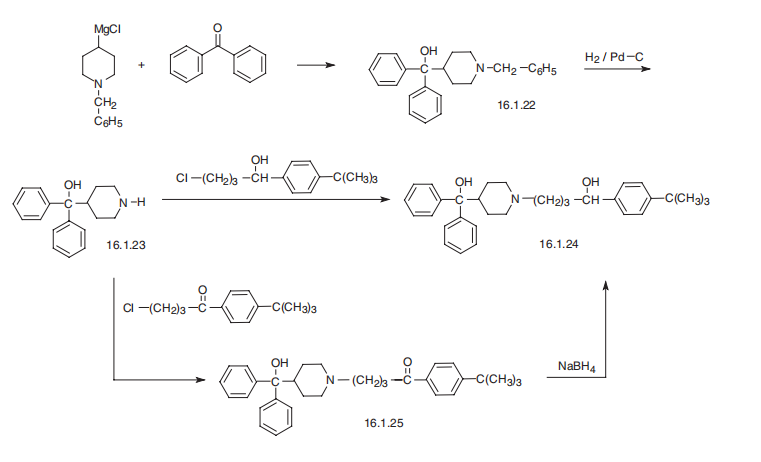
Terfenadine, |á-(4-tert-butylphenyl) -4-hydroxydiphenylmethyl)- 1-piperidinebutanol (16.1.24), is synthesized in two ways. According to the first, benzyl-4- magnesiumchloropiperidine is reacted with benzophenone, giving (1-benzyl-4-piperidyl) diphenylcarbinol (16.2.22), which undergoes further debenzylation by reduction with hydrogen using a palladium over carbon catalyst, giving (4-piperidyl)diphenylcarbinol (16.2.22). This product is alkylated by either 1-(4-tert-butylphenyl)-4-chlorobutanol, which forms terfenadine (16.1.24), or by alkylating with (4-tert-butylphenyl-3-chloropropiophenone, which forms the product (16.1.25), the carbonyl group of which is reduced to an alcohol group, thus giving the desired terfenadine (16.1.24).
storage
Store at +4°C
Terfenadine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3581 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9320 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29220 | 58 |
View Lastest Price from Terfenadine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-11-24 | Terfenadine
50679-08-8
|
US $0.00-0.00 / Kg/Drum | 1KG | 98%min | 500KG | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2021-07-20 | Terfenadine
50679-08-8
|
US $1.00-1.00 / KG | 1g | 99% | 50tons | Shaanxi Dideu Medichem Co. Ltd | |
 |
2021-06-17 | Terfenadine USP/EP/BP
50679-08-8
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited |
-

- Terfenadine
50679-08-8
- US $0.00-0.00 / Kg/Drum
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Terfenadine
50679-08-8
- US $1.00-1.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd
-

- Terfenadine USP/EP/BP
50679-08-8
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited