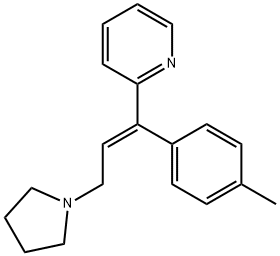
Triprolidine
- CAS No.
- 486-12-4
- Chemical Name:
- Triprolidine
- Synonyms
- Triprolidin;NCI-C61450;(e)-pyridin;TRIPROLIDINE;Tripyrolidine;Triprolidine USP/EP/BP;(E)-1-(2-pyridyl)-3-pyrrolidin-1-yl-1-p-tolylpropene;trans-1-(2-Pyridyl)-3-pyrrolidino-1-p-tolylprop-1-ene;trans-2-[3-(1-Pyrrolidinyl)-1-p-tolypropenyl]pyridine;(E)-2-[3-(1-Pyrrolidinyl)-1-p-toluenepropenyl]pyridine
- CBNumber:
- CB7782853
- Molecular Formula:
- C19H22N2
- Molecular Weight:
- 278.39
- MDL Number:
- MFCD00038040
- MOL File:
- 486-12-4.mol
- MSDS File:
- SDS
| Melting point | 59-61° |
|---|---|
| Boiling point | 435.4±34.0 °C(Predicted) |
| Density | 1.061±0.06 g/cm3(Predicted) |
| pka | pKa 4.01(H2O t = 23.0) (Uncertain);9.69(H2O t = 23.0) (Uncertain) |
| CAS DataBase Reference | 486-12-4(CAS DataBase Reference) |
| FDA UNII | 2L8T9S52QM |
| NIST Chemistry Reference | Triprolidine(486-12-4) |
| ATC code | R06AX07 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
Triprolidine price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| AK Scientific | N001 | Triprolidine | 486-12-4 | 1g | $890 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0004520 | TRIPROLIDINE 95.00% | 486-12-4 | 100MG | $902.15 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0004520 | TRIPROLIDINE 95.00% | 486-12-4 | 1G | $1882.88 | 2021-12-16 | Buy |
Triprolidine Chemical Properties,Uses,Production
Originator
Actidil,Burroughs-Wellcome,US,1958
Uses
Antihistaminic.
Definition
ChEBI: An N-alkylpyrrolidine that is acrivastine in which the pyridine ring is lacking the propenoic acid substituent. It is a sedating antihistamine that is used (generally as the monohydrochloride monohydrate) for the relief of the symptoms o uticaria, rhinitis, and various pruritic skin disorders.
Manufacturing Process
4-Methylacetophenone is first reacted with paraformaldehyde and then with
pyrrolidine to give p-methyl-ω-pyrralidinopropiophenone.
Atomized lithium (26 g, 3.75 mols) and sodium-dried ether (200 cc) are
placed in a 3-liter, 3-necked flask fitted with a Herschberg stirrer,
thermometer pocket and a water condenser closed by a calcium chloride tube.
A slow stream of dry nitrogen is blown through the flask, which is cooled to -
10°C and n-butyl chloride (138 g, 156 cc, 1.5 mols) is run in with rapid
stirring; the mixture is stirred for a further 30 minutes, and then cooled to -
60°C
2-Bromopyridine (193 g, 1.22 mols) is then added dropwise over 20 minutes,
the temperature of the reaction mixture being maintained at -50°C. The
mixture is stirred for 10 minutes at -50°C and p-methyl-ω-
pyrrolidinopropiophenone (112.5 g, 0.5 mol) in dry benzene is then added
dropwise over ca 30 minutes, at a temperature of -50°C. The mixture is
stirred for a further 2 hours, the temperature being allowed to rise to -30°C
but no higher.
The mixture is poured onto excess ice, acidified with concentrated
hydrochloric acid, the ether layer separated and extracted with water (1 x 200
cc). The combined aqueous extracts are washed with ether (1 x 200 cc)
basified with 0.880 ammonia and extracted with chloroform (3 x 350 cc); the
extract is washed with water (2 x 100 cc), dried over sodium sulfate,
evaporated, and the residue extracted with boiling light petroleum (BP 60° to
80°C; 10 volumes), filtered hot and evaporated to dryness. The residue is
recrystallized from alcohol to give a cream solid (119 g, 80%), MP 117° to
118°C. Recrystallization gives 1-(4-methylphenyl)-1-(2-pyridyl)-3-
pyrrolidonopropan-1-ol, MP 119° to 120°C.
1-(4-Methylphenyl)-1-(2-pyridyl)-3-pyrrolidinopropan-1-ol (10.0 g) is heated
in a steam bath for 30 minutes with 85% aqueous sulfuric acid (30 cc). The
solution is then poured onto crushed ice, excess of ammonia solution added
and the liberated oil extracted with light petroleum (BP 60° to 80°C). The
extract is dried over anhydrous sodium sulfate and the solvent evaporated to
leave an amber syrup (8.8 g) consisting of the cis and trans isomers of 1-(4-
methylphenyl)-1-(2-pyridyl)-3-pyrrolidinoprop-1-ene as described in US Patent
2,712,023. The isomers may be separated by base exchange chromatography.
The 4-methyl-ω-pyrrolidinopropiophenone required as the starting product for the preparation of the carbinol is prepared by the Mannich reaction (Blicke,
Organic Reactions, 1942, vol 1, p 303; Adamson & Billinghurst, Journal of the
Chemical Society, 1950,1039) from 4-methylacetophenone and pyrrolidine.
The hydrochloride has a MP of 170°C with decomposition.
brand name
Actidil (GlaxoSmithKline); Myidyl (USl).
Therapeutic Function
Antihistaminic
Triprolidine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Franta Biotechnology Co., Ltd | +86-13082019107 +86-13082019107 | admin@flanderff.com | China | 229 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3683 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29322 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| Hong Kong Excellence Biotechnology Co., Ltd. | +86-86-18838029171 +8618126314766 | ada@sh-teruiop.com | China | 893 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9165 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
View Lastest Price from Triprolidine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-27 | Triprolidine
486-12-4
|
US $20.00 / kg | 1kg | 99% | 1000kg | Shaanxi Franta Biotechnology Co., Ltd | |
 |
2021-07-20 | Triprolidine
486-12-4
|
US $1.00-1.00 / KG | 1g | 99% | 50tons | Shaanxi Dideu Medichem Co. Ltd |
-

- Triprolidine
486-12-4
- US $20.00 / kg
- 99%
- Shaanxi Franta Biotechnology Co., Ltd
-

- Triprolidine
486-12-4
- US $1.00-1.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd
486-12-4(Triprolidine)Related Search:
1of4