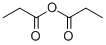
Propionic anhydride
- CAS No.
- 123-62-6
- Chemical Name:
- Propionic anhydride
- Synonyms
- propionic;Propionic acid anhydride;PROPANOIC ANHYDRIDE;propionyloxide;Propionyl oxide;C2H5C(O)OC(O)C2H5;Propinoicanhydride;Propanic Anhydride;Propionic Anhydried;PROPIONIC ANHYDRIDE
- CBNumber:
- CB7852974
- Molecular Formula:
- C6H10O3
- Molecular Weight:
- 130.14
- MDL Number:
- MFCD00009303
- MOL File:
- 123-62-6.mol
- MSDS File:
- SDS
| Melting point | -42 °C |
|---|---|
| Boiling point | 167 °C(lit.) |
| Density | 1.015 g/mL at 25 °C(lit.) |
| vapor density | 4.5 (vs air) |
| vapor pressure | 10 mm Hg ( 57.7 °C) |
| refractive index |
n |
| Flash point | 165 °F |
| storage temp. | Store below +30°C. |
| solubility | H2O: decomposes (when in contact with water) |
| form | clear liquid |
| color | Colorless to Almost colorless |
| Odor | like acetaldehyde |
| PH | 2.5 (100g/l, H2O, 20℃)(calculated on the free acid) |
| explosive limit | 1.3-9.5%(V) |
| Water Solubility | hydrolyses |
| Sensitive | Moisture Sensitive |
| Merck | 14,7826 |
| BRN | 507066 |
| Dielectric constant | 18.0(16℃) |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, water, moisture, most common metals, active halogen compounds, ammonia, amines. |
| LogP | 0.33 at 20℃ |
| CAS DataBase Reference | 123-62-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | E3A2VV18E6 |
| NIST Chemistry Reference | Propanoic acid, anhydride(123-62-6) |
| EPA Substance Registry System | Propionic anhydride (123-62-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314 | |||||||||
| Precautionary statements | P280-P301+P330+P331-P303+P361+P353-P304+P340+P310-P305+P351+P338-P363 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 34-R34 | |||||||||
| Safety Statements | 26-45-S45-S26 | |||||||||
| RIDADR | UN 2496 8/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | UF9100000 | |||||||||
| Autoignition Temperature | 545 °F | |||||||||
| Hazard Note | Corrosive/Moisture Sensitive | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2915 90 70 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 orally in rats: 2.36 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Propionic anhydride price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.00608 | Propionic anhydride for synthesis | 123-62-6 | 100mL | $27 | 2024-03-01 | Buy |
| Sigma-Aldrich | 81942 | Propionic anhydride purum, ≥96.0% (NT) | 123-62-6 | 1l | $81.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00608 | Propionic anhydride for synthesis | 123-62-6 | 500mL | $47.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00608 | Propionic anhydride for synthesis | 123-62-6 | 1L | $78.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 240311 | Propionic anhydride ≥99% | 123-62-6 | 50g | $59.5 | 2024-03-01 | Buy |
Propionic anhydride Chemical Properties,Uses,Production
Chemical Properties
Propionic anhydride is a colorless liquid. Strong, pungent, unpleasant odor. Soluble in methanol, ethanol, ether, chloroform and alkali, decomposes in water.
Uses
Propionic anhydride is used as a esterifying agent for certain perfume oils, fats, oils, and especially cellulose and as a reagent in organic synthesis. In the production of alkyd resins, dyestuffs and drugs. Has been used as a dehydrating agent in some sulfonations and nitrations.
Application
Propionic anhydride was previously used in the preparation of α and β-1-propionyl derivatives of glucopyranose tetra-acetate.
General Description
Propionic anhydride is an organic compound with the molecular formula (CH3CH2CO)2O. it is an colorless acid anhydride that is widely used as a reagent in organic synthesis.
Air & Water Reactions
Decomposes exothermically in water to form a corrosive solution of propionic acid [Merck, 11th ed. 1989].
Reactivity Profile
Propionic anhydride reacts exothermically with water. The reactions are sometimes slow, but can become violent when local heating accelerates their rate. Acids accelerate the reaction with water. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases.
Hazard
Strong irritant to tissue.
Health Hazard
Inhalation causes irritation of eyes and respiratory tract. Contact with liquid causes burns of eyes and skin. Ingestion causes burns of mouth and stomach.
Fire Hazard
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic by ingestion. Mildly toxic by skin contact. A corrosive irritant to skin, eyes, and mucous membranes. Combustible when exposed to heat or flame; can react with oxidizing materials. To fight fire, use CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. Used as an esterifyng agent and dehydrating agent. See also ANHYDRIDES.
Safety
Propanoic anhydride is strong smelling and corrosive, and will cause burns on contact with skin. Vapour can burn eyes and lungs.
Synthesis
Propanoic anhydride has been prepared by dehydration of propanoic acid using ketene :
2 CH3CH2CO2H + CH2= C= O → (CH3CH2CO)2O + CH3CO2H.
Potential Exposure
Used in the manufacture of perfumes, flavorings, alkyd resins; dyestuffs, pharmaceuticals; as an esterifying agent for fats, oils, and cellulose; dehydrating medium for nitrations and sulfonations.
Shipping
UN2496 Propionic anhydride, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Shake the anhydride with P2O5 for several minutes, then distil. [Beilstein 2 IV 722.]
Incompatibilities
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, perox- ides, permanganates, perchlorates, chlorine, bromine, fluo- rine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, reducing agents; alcohols and metals. Contact with water forms heat 1 flammable propionic acid. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercap- tans, nitrides, and sulfides (releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur).
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Propionic anhydride Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 | info@longyupharma.com | China | 2531 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Richest Group Ltd | 18017061086 | oled@richest-group.com | CHINA | 5601 | 58 |
| Hebei Binshare New Material Co. Ltd | +8618633865755 | china01@hbbinshare.com | CHINA | 969 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
Related articles
- What is Propionic anhydride?
- Propionic anhydride is used as a propionylating agent in the manufacture of medicines, fragrances and special esters. It is us....
- Sep 8,2021
View Lastest Price from Propionic anhydride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-14 | Propionic anhydride
123-62-6
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-08-16 | Propionic anhydride
123-62-6
|
US $80.00 / KG | 1KG | >99% | 20tons | Weijer International Trade (Hebei) Co., Ltd | |
 |
2023-06-26 | Propionic anhydride
123-62-6
|
US $150.00 / ASSAYS | 10g | >99% | 500kg/month | Hebei Mingeng Biotechnology Co., Ltd |
-

- Propionic anhydride
123-62-6
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Propionic anhydride
123-62-6
- US $80.00 / KG
- >99%
- Weijer International Trade (Hebei) Co., Ltd
-

- Propionic anhydride
123-62-6
- US $150.00 / ASSAYS
- >99%
- Hebei Mingeng Biotechnology Co., Ltd
123-62-6(Propionic anhydride)Related Search:
1of4