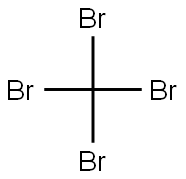
Carbon tetrabromide
- CAS No.
- 558-13-4
- Chemical Name:
- Carbon tetrabromide
- Synonyms
- CBr4;TETRABROMOMETHANE;PERBROMOMETHANE;TETRABROMOETHANE;carbonbromide;TetrabroMoMeth;Carbon bromide;bromiduhlicity;Bromid uhlicity;Tetrabrommethan
- CBNumber:
- CB7853830
- Molecular Formula:
- CBr4
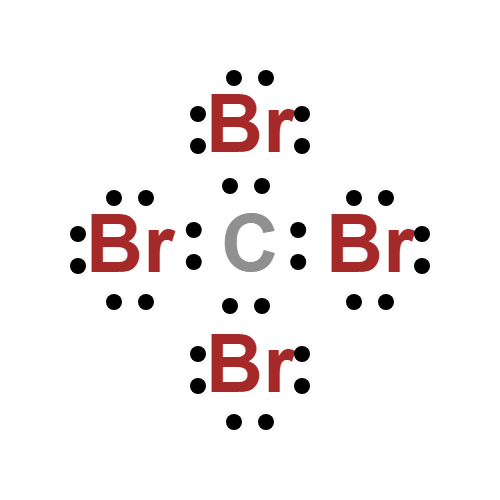
Lewis structure
- Molecular Weight:
- 331.63
- MDL Number:
- MFCD00000117
- MOL File:
- 558-13-4.mol
- MSDS File:
- SDS
| Melting point | 88-90 °C(lit.) |
|---|---|
| Boiling point | 190 °C(lit.) |
| Density | 3,42 g/cm3 |
| vapor pressure | 40 mm Hg ( 96 °C) |
| refractive index | 1.5942 |
| Flash point | 190°C |
| storage temp. | Store below +30°C. |
| solubility | soluble in Chloroform |
| form | Crystalline Solid |
| color | White to off-white |
| Water Solubility | insoluble |
| BRN | 1732799 |
| Dielectric constant | 7(22.0℃) |
| Exposure limits |
ACGIH: TWA 0.1 ppm; STEL 0.3 ppm NIOSH: TWA 0.1 ppm(1.4 mg/m3); STEL 0.3 ppm(4 mg/m3) |
| CAS DataBase Reference | 558-13-4(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | NLH657095L |
| NIST Chemistry Reference | Carbon tetrabromide(558-13-4) |
| EPA Substance Registry System | Carbon tetrabromide (558-13-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H315-H318-H335 | |||||||||
| Precautionary statements | P261-P264-P280-P301+P312-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,T+,N,Xn | |||||||||
| Risk Statements | 37/38-41-36-26-52/53-22 | |||||||||
| Safety Statements | 26-36-45-27-24-61 | |||||||||
| RIDADR | UN 2516 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | FG4725000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29033036 | |||||||||
| NFPA 704 |
|
Carbon tetrabromide price More Price(41)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | C11081 | Tetrabromomethane ReagentPlus , 99% | 558-13-4 | 5g | $25.08 | 2024-03-01 | Buy |
| Sigma-Aldrich | C11081 | Tetrabromomethane ReagentPlus , 99% | 558-13-4 | 100g | $81.9 | 2024-03-01 | Buy |
| TCI Chemical | T0038 | Carbon Tetrabromide >99.0%(GC) | 558-13-4 | 25g | $24 | 2024-03-01 | Buy |
| TCI Chemical | T0038 | Carbon Tetrabromide >99.0%(GC) | 558-13-4 | 100g | $66 | 2024-03-01 | Buy |
| Alfa Aesar | L00821 | Carbon tetrabromide, 98% (dry wt.), may cont. up to ca 6% water | 558-13-4 | 25g | $33.65 | 2024-03-01 | Buy |
Carbon tetrabromide Chemical Properties,Uses,Production
Description
Carbon tetrabromide is considered a highly toxic chemical, may be fatal if inhaled, swallowed, or absorbed through skin. It is metabolized in vitro to produce carbon monoxide but the in vivo significance has not been established. Under anaerobic reducing conditions it forms complexes with ferrous cytochrome P450. Carbon monoxide is detected as a metabolic product of the interaction. Carbon tetrabromide’s production and use in organic syntheses may result in its release to the environment through various waste streams. Carbon tetrabromide has been isolated from red algae, Asparagopsis toxiformis, found in the ocean near Hawaii. It was detected in water from treated chlorinated seawater used for drinking at oil platforms. Occupational exposure to carbon tetrabromide may occur through inhalation and dermal contact with this compound at workplaces where it is produced or used. The general population may be exposed to carbon tetrabromide via ingestion of drinking water. Acute exposures to high concentrations may cause upper respiratory tract irritation and injury to lungs, liver (hepatotoxicity) and kidneys (nephrotoxicity). Chronic exposure effects at very low levels will be almost entirely limited to liver injury. It is a potent lachrymator even at low exposure concentrations. Although carbon tetrabromide may release bromine ions during metabolism, clinical bromism is not expected to occur.
Chemical Properties
Crystalline colorless
Chemical Properties
Carbon tetrabromide, is a colorless powder, white crystalline solid, or yellow-brown crystals. Slight odor
Uses
Used to a limited extent as an intermediate in organic synthesis
Uses
Organic synthesis.
Uses
Carbon tetrabromide is used to a limited extent as a chemical intermediate. It has been isolated from red algae, Asparagopsis toxiformis, found in the ocean near Hawaii.
Definition
ChEBI: A one-carbon compound substituted by 4 bromo groups.
General Description
A colorless crystalline solid. Much more dense than water and insoluble in water. Toxic by ingestion. Vapors are narcotic in high concentration. Used to make other chemicals.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Carbon tetrabromide is incompatible with the following: Strong oxidizers, hexacyclohexyldilead, lithium .
Hazard
A poison; narcotic in high concentration. Liver damage, eye, skin, and upper respiratory tract irritant.
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Safety Profile
Poison by subcutaneous and intravenous routes. Narcotic in high concentration. Mixture with Li particles is an impact-sensitive explosive. Explodes on contact with hexacyclohexylddead. When heated to decomposition it emits toxic fumes of Br-. See also CHLORINATED HYDROCARBONS, ALIPHATIC.
Potential Exposure
CBr4 is used in organic synthesis.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
Environmental Fate
Carbon tetrabromide inhibits protein synthesis and causes lipid peroxidation, both of which may be involved in cell injury or death mediated by free radicals.
storage
Color Code—Green: General storage may be used.Prior to working with carbon tetrabromide you should betrained on its proper handling and storage. Store in tightlyclosed containers in a cool, well-ventilated area away fromoxidizers and other incompatible materials listed above.
Shipping
UN2516 Carbon tetrabromide, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Reactive bromide is removed from CBr4 by refluxing with dilute aqueous Na2CO3, then steam distilling, crystallising from EtOH, and drying in the dark under vacuum. [Sharpe & Walker J Chem Soc 157 1962.] It can be sublimed at 70o and low pressure. [Beilstein 1 IV 85.]
Toxicity evaluation
Carbon tetrabromide is a colorless nonflammable solid at room temperature. It is insoluble in water, but soluble in several organic solvents such as alcohol, ether, and chloroform. Its specific gravity is 3.42, melting point is 90°C, boiling point is 189°C, and vapor pressure is 0.72 torr at 25°C. Production and use of carbon tetrabromide may result in its release in the environment through various hazardous waste streams. Carbon tetrabromide is expected to have very high mobility in soil and volatilizes slowly from dry soil surface. Its biodegradation is expected to be slow and to exist solely as a vapor in the ambient atmosphere. It is not expected to adsorb to suspended solids and sediment in the water column. Its potential for bioconcentration in aquatic organisms is moderate.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, lithium and hexacyclohexyldiilead, since violent reactions may occur.
Waste Disposal
Purify by distillation and return to suppliers.
Carbon tetrabromide Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23556 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12457 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7613 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
Related articles
- The applications of carbon tetrabromidein organic synthesis
- carbon tetrabromide (CBr4) is the simplest (tetrahedral) building element.
- Jun 6,2022
View Lastest Price from Carbon tetrabromide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-12-23 | Carbon tetrabromide
558-13-4
|
US $50.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-09-06 | Carbon tetrabromide
558-13-4
|
US $0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2023-08-02 | Carbon tetrabromide
558-13-4
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |
-

- Carbon tetrabromide
558-13-4
- US $50.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Carbon tetrabromide
558-13-4
- US $0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
-

- Carbon tetrabromide
558-13-4
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd