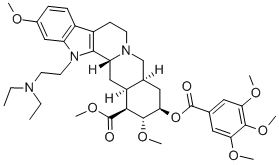
bietaserpine
- CAS No.
- 53-18-9
- Chemical Name:
- bietaserpine
- Synonyms
- S-1210;DL-152;bietaserpine;Bietasperine;Bietaserpinum;Diethylaminoreserpine;(3β,20α)-1-[2-(Diethylamino)ethyl]-11,17α-dimethoxy-18β-[(3,4,5-trimethoxybenzoyl)oxy]yohimban-16β-carboxylic acid methyl;Yohimban-16-carboxylic acid, 1-[2-(diethylamino)ethyl]-11,17-dimethoxy-18-[(3,4,5-trimethoxybenzoyl)oxy]-, methyl ester, (3β,16β,17α,18β,20α)-
- CBNumber:
- CB7901926
- Molecular Formula:
- C39H53N3O9
- Molecular Weight:
- 707.86
- MDL Number:
- MFCD00242906
- MOL File:
- 53-18-9.mol
| alpha | D17 -121° (c = 2 in CHCl3) |
|---|---|
| Boiling point | 706.92°C (rough estimate) |
| Density | 1.1581 (rough estimate) |
| refractive index | 1.7800 (estimate) |
| FDA UNII | 0P5B94FVD5 |
| ATC code | C02AA07,C02AA57 |
bietaserpine Chemical Properties,Uses,Production
Originator
Tensibar,Lefranco,France,1967
Definition
ChEBI: (1R,15S,17R,18R,19S,20S)-3-[2-(diethylamino)ethyl]-6,18-dimethoxy-17-[oxo-(3,4,5-trimethoxyphenyl)methoxy]-11,12,14,15,16,17,18,19,20,21-decahydro-1H-yohimban-19-carboxylic acid methyl ester is a yohimban alkaloid.
Manufacturing Process
The first stage is to prepare the naphthyl sodium solution in the following
way:
To a solution of 0.6 g naphthalene in 10 ml tetrahydrofurane, anhydrous, used
as solvent, add 96 mg sodium under a nitrogen atmosphere. After a few
minutes, an intensive dark green coloration develops, while the sodium
dissolves. The reaction is completed after a period of time ranging between 30
and 60 minutes.
Then add to the above solution a solution of 2.42 g reserpine in 60 ml
anhydrous dioxan at 50°C.
After heating for 15 minutes (which corresponds to carrying out reaction a),
add 0.6 g, diethylaminochloroethane, while the mixture is kept boiling under
reflux, for 6 hours. Reaction b is then completed.
Then cool the mixture and evaporate the dioxan under reduced pressure. The
pasty residue is dissolved in a mixture of 50 ml benzene and 20 ml ether, and
washed several times with water.
The aqueous solutions resulting from the washing are also extracted with ether, and the ether portions are added to the main ether-benzene solution.
This solution is extracted several times with 5% acetic acid, until the silicotungstate
test (an identification test for alkaloids) yields a negative result, and
the acetic solutions are washed with 10 ml ether.
After combining the acetic extracts, the solution is adjusted to a pH of 9 with
sodium carbonate, which precipitates the base, which is insoluble in water.
The oily suspension obtained in this way is extracted several times with
chloroform. The chloroform solutions are then washed, each with 10 ml water,
then they are combined and dried over anhydrous potassium carbonate.
After filtering and evaporating the solvent under reduced pressure, the pasty
residue, constituted by the enriched product, is diluted with 30 ml ether and
in this way 0.225 g reserpine (which has not taken part in the reaction) is
isolated by filtration.
After evaporation of the ether under reduced pressure, 1.525 g of the crude
resinous base is obtained, which constitutes the required product in a crude
and impure condition.
This product is purified in the following way: After dissolving in 15 ml of dry
benzene, the resulting solution is filtered on an alumina column, which fixes
the base.
After consecutive elutions with pure benzene, and benzene containing
increasing proportions of chloroform,0.748 g of 1-diethylaminoethylreserpineis
isolated in the form of a resin. The crystalline acid bitartrate
prepared in ethyl acetate melts at 145°-150°C, with decomposition.
Therapeutic Function
Antihypertensive
bietaserpine Preparation Products And Raw materials
bietaserpine Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 | support@targetmol.com | United States | 19973 | 58 |
| Organtec Ltd | 010-89719903 18511074324 | danlismi@outlook.com | China | 1334 | 58 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 | 1046@dideu.com | China | 10008 | 58 |
| TargetMol Chemicals Inc. | 4008200310 | marketing@tsbiochem.com | China | 24131 | 58 |
| Supplier | Advantage |
|---|---|
| TargetMol Chemicals Inc. | 58 |
| Organtec Ltd | 58 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 58 |
| TargetMol Chemicals Inc. | 58 |
53-18-9(bietaserpine )Related Search:
1of4