Basifungin
- CAS No.
- 127785-64-2
- Chemical Name:
- Basifungin
- Synonyms
- LY-295337;Basifungin;aureobasidin A;Aureobasidin A (AbA);Aureobasidin A (9CI);Basifungin/Aureobasidin A;Aureobasidin A(Basifungin);Basifungin ISO 9001:2015 REACH;Aureobasidin A (9CI) CAS NO.127785-64-2;Aureobasidin A,ceramide,inositolphosphorylceramides,Fungal,Inhibitor,intoxication,inhibit,Basifungin
- CBNumber:
- CB81120091
- Molecular Formula:
- C60H92N8O11
- Molecular Weight:
- 1101.42
- MDL Number:
- MFCD01675341
- MOL File:
- 127785-64-2.mol
- MSDS File:
- SDS
| Boiling point | 1229.1±65.0 °C(Predicted) |
|---|---|
| Density | 1.19±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C, protect from light |
| solubility | DMSO : 100 mg/mL (90.79 mM; Need ultrasonic) |
| pka | 13.35±0.70(Predicted) |
| FDA UNII | VV0USO6I6U |
Basifungin Chemical Properties,Uses,Production
Description
Aureobasidin A (R-106; Basifungin), the lead compound, has potent activity in vitro against many clinically relevant fungi, including Candida spp. , Cryptococcus neoformans, Blastomyces dermatidis, Histoplasma capsulatum, and some Aspergillus species. In vivo, in a murine model of systemic candidiasis, the compound was well tolerated and more effective than AmB and fluconazole. Molecular studies of aureobasidin A-resistant mutants of S. cerevisiae have led to the discovery of the aureobasidin resistance (AUR1?) gene. It has been suggested that the gene product of the wild-type AUR1+ is the molecular target for aureobasidin A. AUR1+ can fully restore the activity of IPC synthase and sphingolipid synthesis, and that aureobasidin A does indeed function as a potent inhibitor of IPC synthase. The inhibition of this essential biosynthetic step leads to ceramide accumulation in growing cells and cell death. Cytological studies in S. cerevisiae after aureobasidin A exposure and after disruption of the AUR1+ gene suggest that the ultimate effect of the compound is the destruction of the cell membrane and the disturbance of intracellular microtubule organization[2].
Uses
Basifungin, also known as aureuobacidin A, is an orally available cyclic depsipeptide antibiotic produced by Aureobasidium pullulans that is a fungicide at low concentrations. Aureobasidin A inhibits inositol phosphorylceramide synthase, an enzyme that catalyzes a key step in fungal sphingolipid biosynthesis. This may inhibit fungal cell growth.
Definition
ChEBI: Basifungin is a cyclodepsipeptide antibiotic, which is isolated from the filamentous fungus Aureobasidium pullulans R106 and is toxic to yeast at low concentrations (0.1-0.5 ug/ml).
Biological Activity
Aureobasidin A (AbA; Basifungin) is an antibiotic that inhibits the synthesis of the plant-type sphingolipid inositol phosphorylceramide (IPC). Basifungin treatment inhibited T. gondii replication irreversibly. More importantly, AbA treatment did not induce stage conversion to the bradyzoite form, emphasizing the parasitocidal effect of the compound. This was further confirmed by AbA-induced morphological alterations in the parasite cell shape and integrity, with prominent cell vacuolization. In addition, AbA affected total sphingolipid neosynthesis in T. gondii and, in particular, increased the relative level of ceramide, the direct precursor of IPC, without causing any modification in the sphingolipid profile of the host cell[1].
Clinical Use
Aureobasidin A (Basifungin) is a cyclic depsipeptide that is produced byfermentation in cultures of Aureobasidium pullulan.Aureobasidin A acts as a tight-binding noncompetitive inhibitorof the enzyme inositol phosphorylceramide synthase(IPC synthase), which is an essential enzyme for fungalsphingolipid biosynthesis. A unique structural feature of theaureobasidins is the N-methylation of four of seven amide nitrogenatoms. The lack of tautomerism dictated by N-methylationmay contribute to forming a stable solution conformerthat is shaped somewhat like an arrowhead, the presumed biologicallyactive conformation of aureobasidin-A.The pradimycins and benanomycins are naphthacenequinonesthat bind mannan in the presence of Ca2+ to disrupt the cell membrane in pathogenic fungi. Both demonstrate good in vitro and in vivo activity againstCandida spp. and C. neoformans clinical isolates.
References
[1] Sabrina Sonda, Adrian B Hehl. “Lipid biology of Apicomplexa: perspectives for new drug targets, particularly for Toxoplasma gondii.” Trends in parasitology 22 1 (2006): 41–7.
[2] A H Groll, T J Walsh, A J De Lucca. “Emerging targets for the development of novel antifungal therapeutics.” Trends in Microbiology 6 3 (1998): 117–24.
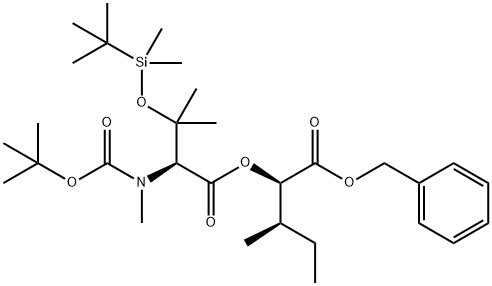
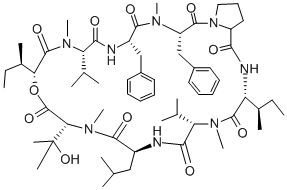
Basifungin Preparation Products And Raw materials
Basifungin Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Minbiotech Co., Ltd. | +8617315815539 | sales@minbiotech.com | CHINA | 129 | 58 |
| Fuxin Pharmaceutical | +86-021-021-50872116 +8613122107989 | contact@fuxinpharm.com | China | 10297 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9350 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | admin@nexconn.com | China | 10248 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| SHANGHAI T&W PHARMACEUTICAL CO., LTD. | +86-021-61551413 +8618813727289 | contact@trustwe.com | China | 5738 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
| Zhejiang Huida Biotech Co., LTD | 008613515763466 8615669048680 | wendy@huidabiotech.com | CHINA | 87 | 58 |
Related articles
- Uses of aureobasidin A
- Analogs of khafrefungin were designed and synthesized but none had increased potency and most had lost antifungal activity. Ru....
- Apr 1,2022





