Medroxyprogesterone Acetate
- CAS No.
- 71-58-9
- Chemical Name:
- Medroxyprogesterone Acetate
- Synonyms
- MEDROXYPROGESTERONE 17-ACETATE;PROVERA;Medroxyprogest;Amen;MEDROXYPROGESTRONE ACETATE;Medroxyprogesterone acetate for system suitability CRS;u8839;Nidaxin;oragest;veramix
- CBNumber:
- CB8130110
- Molecular Formula:
- C24H34O4
- Molecular Weight:
- 386.52
- MDL Number:
- MFCD00010483
- MOL File:
- 71-58-9.mol
- MSDS File:
- SDS
| Melting point | 206-207 °C(lit.) |
|---|---|
| alpha | D +61° (in chloroform) |
| Boiling point | 432.7°C (rough estimate) |
| Density | 1.0346 (rough estimate) |
| refractive index | 48 ° (C=1, Dioxane) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Practically insoluble in water, freely soluble in methylene chloride, soluble in acetone, sparingly soluble in ethanol (96 per cent) |
| color | White |
| Water Solubility | <0.1 g/100 mL at 23 ºC |
| Merck | 13,5817 |
| BRN | 2066112 |
| BCS Class | 2,3,1 |
| Stability | Stable, but weakly air and light sensitive. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 71-58-9(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | medroxyprogesterone acetate |
| FDA UNII | C2QI4IOI2G |
| NCI Drug Dictionary | Amen |
| Proposition 65 List | Medroxyprogesterone Acetate |
| IARC | 2B (Vol. 21, Sup 7) 1987 |
| EPA Substance Registry System | Medroxyprogesterone acetate (71-58-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H351-H413 |
| Precautionary statements | P201-P202-P273-P280-P308+P313-P405 |
| Hazard Codes | Xn |
| Risk Statements | 40-48 |
| Safety Statements | 22-36/37/39-45 |
| WGK Germany | 3 |
| RTECS | TU5010000 |
| HS Code | 29372390 |
Medroxyprogesterone Acetate price More Price(30)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 46412 | Medroxyprogesterone 17-acetate VETRANAL | 71-58-9 | 250mg | $74.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1378001 | Medroxyprogesterone acetate United States Pharmacopeia (USP) Reference Standard | 71-58-9 | 200mg | $436 | 2024-03-01 | Buy |
| Cayman Chemical | 23664 | Medroxyprogesterone 17-acetate ≥98% | 71-58-9 | 500mg | $62 | 2024-03-01 | Buy |
| Cayman Chemical | 23664 | Medroxyprogesterone 17-acetate ≥98% | 71-58-9 | 1g | $115 | 2024-03-01 | Buy |
| Sigma-Aldrich | Y0001484 | Medroxyprogesterone acetate for peak identification European Pharmacopoeia (EP) Reference Standard | 71-58-9 | y0001484 | $153 | 2024-03-01 | Buy |
Medroxyprogesterone Acetate Chemical Properties,Uses,Production
Description
Medroxyprogesterone Acetate, also known as Medroxyprogesterone 17-acetate or MPA, is a synthetic progestogen and a steroidal progestin. It is derived from the human hormone progesterone. It prevents fertilization and increases the rate of transport of eggs from the fallopian tubes to the uterus in female ferrets when administered prior to ovulation. Medroxyprogesterone 17-acetate reversibly blocks ovulation in rats when injected on the last day of diestrus. It also has anti-androgenic activity in rats, decreasing plasma testosterone levels via induction of hepatic testosterone reductase activity. Medroxyprogesterone 17-acetate exhibits immunosuppressive effects in vitro and in vivo, inhibiting the production of IFN-γ by CD2/CD3/CD28-stimulated peripheral blood mononuclear cells (PBMCs) at concentrations ≥10 nM and extending the survival of rabbit skin allografts. Injectable formulations containing medroxyprogesterone 17-acetate have been used as contraceptives.
Chemical Properties
White or almost white, crystalline powder.
Originator
Provera,Upjohn,US,1959
Uses
Medroxyprogesterone Acetate is a synthetic progesterone receptor agonist that is used to treat amenorrhea (unusual stopping of menstrual periods) and abnormal uterine bleeding.
Definition
ChEBI: Medroxyprogesterone acetate is an acetate ester resulting from the formal condensation of the 17alpha-hydroxy group of medroxyprogesterone with the carboxy group of acetic acid. A widely used progestin in menopausal hormone therapy and in progestogen-only birth control. It has a role as a progestin, an androgen, a female contraceptive drug, a synthetic oral contraceptive, an adjuvant, an inhibitor, an antioxidant and an antineoplastic agent. It is a steroid ester, an acetate ester, a 20-oxo steroid, a 3-oxo-Delta(4) steroid and a corticosteroid. It is functionally related to a medroxyprogesterone.
brand name
Amen (Amarin); Curretab (Solvay Pharmaceuticals); Cycrin (ESI); Provera (Pharmacia & Upjohn);Clinovie;Cliovir;Dep0-clinover;Dep0-map;Depcorlutin;Depo-prodasone;Depo-progevera;Depo-promone;Deporone;Dugen;Farlurin;Farlutale;Gesinal;Gestapuran;Gestapuron;G-farlutal;Hysron;Intex;Luteocrin orale;Luteodione;Luteos;Lutoporal;Metigestene;Nadigest;Nogest;Onco-provera;Perlutest;Petogen;Piermap;Povera;Promone-e;Pronone;Proverone;Provest;Sindomens;Sodelut "g";Supprestal;Verafen;Veramix plus v.
Therapeutic Function
Progestin
World Health Organization (WHO)
A depot preparation containing 150 mg medroxyprogesterone acetate was introduced over 20 years ago for use as a long-acting injectable contraceptive. Subsequently, positive results of carcinogenicity studies carried out in beagle bitches led to refusal of registration in the United States. These findings were later considered irrelevant to contraceptive use in women and the drug was approved by the Food and Drug Administration. Menstrual irregularities are the most common adverse effect associated with depot medroxyprogesterone acetate. Risk-benefit judgements differ significantly from country to country, having regard to differing national circumstances. The preparation is, however, widely available and is included in the WHO Model List of Essential Drugs. (Reference: (WHTAC4) The Use of Essential Drugs, 4th Report of the WHO Expert Committee, 796, , 1990)
General Description
Medroxyprogesterone acetate is an odorless white to off-white microcrystalline powder. It is a synthetic, acetate derivative of the sex hormone progesterone. (NTP, 1992)
Air & Water Reactions
Medroxyprogesterone 17-acetate is sensitive to prolonged exposure to air and light. Insoluble in water.
Reactivity Profile
Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides.
Hazard
Possible carcinogen.
Fire Hazard
Flash point data for Medroxyprogesterone 17-acetate are not available; however, Medroxyprogesterone 17-acetate is probably combustible.
Biochem/physiol Actions
Medroxyprogesterone 17-acetate (MPA) is a synthetic progestin used as a contraceptive, in hormone replacement therapy and for the treatment of endometriosis. It is a more potent progestin that the nonacetylated form.
Clinical Use
Progestogen:
Cachexia (unlicensed), contraception, epilepsy, male
hypersexuality, malignant neoplasms, respiratory
disorders, sickle-cell disease, dysfunctional uterine
bleeding, endometriosis
Safety Profile
Suspected carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. Human systemic effects by intravenous route: increased intraocular pressure. Human teratogenic effects by an unspecified route: developmental abnormalities of the urogenital system. Human reproductive effects by multiple routes: spermatogenesis, menstrual cycle changes or dlsorders, postpartum effects, female fertility effects, abortion, newborn behavioral effects. Human mutation data reported. Experimental reproductive effects. A drug for the treatment of secondary amenorrhoea and dysfunctional uterine bleeding. When heated to decomposition it emits acrid smoke and irritating fumes.
Veterinary Drugs and Treatments
In cats, MPA has been used when either castration is ineffective or
undesirable to treat sexually dimorphic behavior problems such as
roaming, inter-male aggressive behaviors, spraying, mounting, etc.
MPA has also been used as a tranquilizing agent to treat syndromes
such as feline psychogenic dermatitis and alopecia, but treatment
with “true” tranquilizing agents may be preferable.
In humans, parenteral MPA has been used as a long-acting
contraceptive in females, to decrease sexually deviant behavior in
males, and as an antineoplastic agent for some carcinomas (see
Pharmacology section above). Oral MPA is used in human females
to treat secondary amenorrhea and to treat abnormal uterine bleeding
secondary to hormone imbalances.
Metabolism
Among the first of these substituted 17α-acetoxyprogesterone analogues to be utilized therapeutically was medroxyprogesterone acetate, a 6α-methyl progesterone analogue. This analogue is 25-fold more active than ethisterone. Following oral administration, medroxyprogesterone acetate is completely and rapidly deacetylated by first-pass metabolism to medroxyprogesterone. Medroxyprogesterone is extensively metabolized via pathways similar to those for progesterone, except for 6α-hydroxylation. Most medroxyprogesterone acetate metabolites are excreted in the urine, primarily as glucuronide conjugates. Plasma protein binding for medroxyprogesterone is approximately 86%, primarily to serum albumin, with no binding to SHBG.
References
1. Chang, M.C. Effects of medroxyprogesterone acetate and of ethinyl oestradiol on the fertilization and transportation of ferret eggs. J. Reprod. Fertil. 13(1), 173-174 (1967). DOI:10.1530/JRF.0.0130173
2. Dickmann, Z. Short-and long-term effects of a single injection of depo-medroxyprogesterone acetate (provera) on the vaginal smear, ovulation and mating in the rat. J. Reprod. Fertil. 32(3), 447-451 (1973). DOI:10.1530/JRF.0.0320447
3. Albin, J., Vittek, J., Gordon, G.G., et al. On the mechanism of the anti-androgenic effect of medroxyprogesterone acetate. Endocrinology 93(2), (1973). DOI:10.1210/ENDO-93-2-417
4. Huijbregts, R.P., Michel, K.G., and Hel, Z. Effect of progestins on immunity: Medroxyprogesterone but not norethisterone or levonorgestrel suppresses the function of T cells and pDCs. Contraception 90(2), 123-129 (2014). DOI:10.1016/j.contraception.2014.02.006
5. Turcotte, J.G., Haines, R.F., Brody, G.L., et al. Immunosuppression with medroxyprogesterone acetate. Transplantation 6(2), 248-260 (1968). DOI:10.1097/00007890-196803000-00010
6. Hofmeyr, G.J., Singata-Madliki, M., Lawrie, T.A., et al. Effects of the copper intrauterine device versus injectable progestin contraception on pregnancy rates and method discontinuation among women attending termination of pregnancy services in South Africa: A pragmatic randomized controlled trial. Reprod. Health 13, 42 (2016). DOI:10.1186/s12978-016-0153-9
7. Thomas CP, Liu KZ, Vats HS. Medroxyprogesterone acetate binds the glucocorticoid receptor to stimulate alpha-ENaC and sgk1 expression in renal collecting duct epithelia. Am J Physiol Renal Physiol. 2006 Feb;290(2):F306-12. Epub 2005 Sep 27. DOI:10.1152/AJPRENAL.00062.2005
8. Braden BB, Talboom JS, Crain ID, Simard AR, Lukas RJ, Prokai L, Scheldrup MR, Bowman BL, Bimonte-Nelson HA. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem. 2010Mar;93(3):444-53. DOI:10.1016/j.nlm.2010.01.002
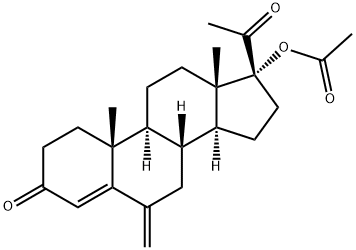
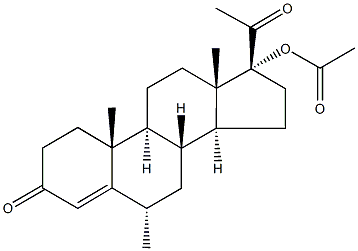
Medroxyprogesterone Acetate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Bonster Technology Co.,Limited | +8613315996897 | bsterltd.wendy@gmail.com | China | 796 | 58 |
| Pharmaceuticals Group Corp., Ltd. | +8613004379774 | wu@jiaerke.com | China | 33 | 58 |
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 | admin01@hsnm.com.cn | China | 2118 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8619133911216 | Jany1001@kangcang.com.cn | China | 338 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| Hong Kong Excellence Biotechnology Co., Ltd. | +86-86-18838029171 +8618126314766 | ada@sh-teruiop.com | China | 892 | 58 |
| Henan Tengmao Chemical Technology Co. LTD | +8615238638457 | salesvip2@hntmhg.com | China | 415 | 58 |
| Wuhan Haorong Biotechnology Co.,ltd | +8618565342920 | sales@chembj.net | China | 269 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
View Lastest Price from Medroxyprogesterone Acetate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-18 | Medroxyprogesterone 17-acetate
71-58-9
|
US $90.00-80.00 / kg | 0.10000000149011612kg | 99% | 100 tons | Hebei Kangcang new material Technology Co., LTD | |
 |
2024-04-18 | Medroxyprogesterone Acetate
71-58-9
|
US $200.00-100.00 / kg | 1kg | 98% | 20 | Hebei Kangcang new material Technology Co., LTD | |
 |
2024-04-18 | Medroxyprogesterone Acetate
71-58-9
|
US $0.00 / G | 1G | 99% | 20 | CONTIDE BIOTECH CO.,LTD |
-

- Medroxyprogesterone 17-acetate
71-58-9
- US $90.00-80.00 / kg
- 99%
- Hebei Kangcang new material Technology Co., LTD
-

- Medroxyprogesterone Acetate
71-58-9
- US $200.00-100.00 / kg
- 98%
- Hebei Kangcang new material Technology Co., LTD
-

- Medroxyprogesterone Acetate
71-58-9
- US $0.00 / G
- 99%
- CONTIDE BIOTECH CO.,LTD
71-58-9(Medroxyprogesterone Acetate)Related Search:
1of4





