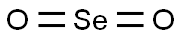Selenium dioxide
- CAS No.
- 7446-08-4
- Chemical Name:
- Selenium dioxide
- Synonyms
- SeO2;SELENIUM(IV) OXIDE;Selenium(IV) dioxide;SELENIUM OXIDE;SELENIUM METAL;SELENIUM STANDARD;YEAST BOUND SELENIUM;VANDEX;NSC 56753;SELENIUM RED
- CBNumber:
- CB8183848
- Molecular Formula:
- O2Se
Lewis structure

- Molecular Weight:
- 110.96
- MDL Number:
- MFCD00003562
- MOL File:
- 7446-08-4.mol
| Melting point | 315 °C (subl.)(lit.) |
|---|---|
| Boiling point | 684.9 °C(lit.) |
| Density | 4.81 g/mL at 25 °C(lit.) |
| vapor pressure | 1 mm Hg ( 157 °C) |
| refractive index | nD20 <1.76 |
| Flash point | 315°C |
| storage temp. | Store below +30°C. |
| solubility | H2O: soluble |
| form | powder |
| color | white |
| Specific Gravity | 3.95 |
| PH | 2 (10g/l, H2O, 20℃) |
| Water Solubility | 38.4 g/100 mL (14 ºC) |
| Sublimation | 315 ºC |
| Merck | 14,8434 |
| Exposure limits |
ACGIH: TWA 0.2 mg/m3 NIOSH: IDLH 1 mg/m3; TWA 0.2 mg/m3 |
| Stability | Stable. Incompatible with organic materials, strong acids, ammonia, nitric acid, halogen acids. Protect from moisture. |
| CAS DataBase Reference | 7446-08-4(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 9N3UK29E57 |
| NIST Chemistry Reference | Selenium dioxide(7446-08-4) |
| EPA Substance Registry System | Selenium dioxide (7446-08-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H331-H373-H410 | |||||||||
| Precautionary statements | P273-P301+P310+P330-P304+P340+P311-P314 | |||||||||
| Hazard Codes | T,N,Xn | |||||||||
| Risk Statements | 36/38-50/53-33-23/25-51/53-20/22 | |||||||||
| Safety Statements | 26-61-60-45-28A-20/21-28 | |||||||||
| RIDADR | UN 3440 6.1/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | VS8575000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2811 29 90 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 orally in Rabbit: 68.1 mg/kg | |||||||||
| NFPA 704 |
|
Selenium dioxide price More Price(38)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.00653 | Selenium dioxide (sublimed) for synthesis | 7446-08-4 | 5G | $34.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00653 | Selenium dioxide (sublimed) for synthesis | 7446-08-4 | 50g | $49.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00653 | Selenium dioxide (sublimed) for synthesis | 7446-08-4 | 250g | $187 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00653 | Selenium dioxide (sublimed) for synthesis | 7446-08-4 | 1kg | $591 | 2024-03-01 | Buy |
| Sigma-Aldrich | 200107 | Selenium dioxide ≥99.9% trace metals basis | 7446-08-4 | 5g | $53.8 | 2024-03-01 | Buy |
Selenium dioxide Chemical Properties,Uses,Production
Physical Properties
White tetragonal crystals; acidic taste; leaves a burning sensation; density 3.95 g/cm3; sublimes at 315°C forming greenish yellow vapors with a sour and pungent odor; melts at 340 to 350°C; vapor pressure 12.5 torr at 70°C; soluble in water, 38.4 g/100mL at 14°C; highly soluble in hot water 82.5 g/100mL at 65°C; soluble in benzene; moderately soluble in ethanol and acetone 6.7 and 4.4g/100mL solvent, respectively, at 15°C; sparingly soluble in acetic acid (1.11g/100mL at 14°C).
Preparation
Selenium dioxide is obtained by burning selenium metal in oxygen:
Se + O2 → SeO2
Selenium also forms a trioxide, SeO3. In excess oxygen the product mixture may contain both dioxide and trioxide. The trioxide is unstable.
Selenium dioxide may be prepared by heating selenium with oxygen and nitrogen dioxide. Presence of excess oxygen would oxidize nitrogen dioxide to pentoxide, instead converting selenium dioxide to trioxide:
2Se + 3O2 + 4NO2 → 2SeO2 + 2N2O5
Selenium dioxide also may be produced by oxidation of selenium by nitric acid. The overall reaction may be written as follows:
Se + 2HNO3 → SeO2 + H2O + NO2 + NO
Reactions
Selenium dioxide is reduced to selenium metal when heated with carbon and other reducing agents.
When heated with ammonia, selenium dioxide forms selenium, nitrogen and water:
3SeO2 + NH3 → 3Se + 2N2 + 6H2O
Ammonia reacts with selenium dissolved in ethanol to form ammonium ethyl selenite, NH4(C2H5)SeO3.
Reaction with nitric acid forms selenic acid:
Se + 2HNO3 → H2SeO4 + 2NO
Selenium dioxide is reduced by hydrazine to black amorphous selenium:
SeO2 + N2H4 → Se + N2 + 2H2O
Hydroxylamine hydrochloride reduces selenium dioxide to reddish-brown amorphous selenium:
SeO2 + 4NH2OH•HCl → Se + 2N2 + 6H2O + 4HCl
The dioxide rapidly absorbs hydrogen halides, forming selenium oxyhalides:
SeO2 + HBr → SeOBr2 + H2O
Reaction with thionyl chloride yields selenium oxychloride:
SeO2 + SOCl2 → SeOCl2 + SO2
Toxicity
The compound is toxic by ingestion. Symptoms of the poisoning effects of selenium dioxide are similar to those of selenium metal. Selenium dioxide vapors are highly irritating to eyes, nose and respiratory tract.
Chemical Properties
Yellowish white to reddish powder or crystals. It is soluble in water and hygroscopic and absorbs moisture or water from the air. Selenium oxide is incompatible with strong oxidising agents, reducing agents, strong acids, ammonia, organics, and phosphorus trichloride.
Chemical Properties
Selenium dioxide is white to slightly reddish crystalline solid or yellow liquid which forms a yellowgreen vapor. It has a sour and pungent odor. Odor threshold in air50.0002 milligram per cubic meter.
Uses
Selenium dioxide (SeO2) is used as an oxidizing agent, as a catalyst, and as an antioxidant for lubricating oils and grease.
Uses
It is used in the manufacture of other selenium compounds. Selenium dioxide is an oxidizing agent mostly used in the allylic oxidation of alkenes to produce allylic alcohols. It is used in creating colorless glass and toner in photographic developing.
Uses
In the manufacture of other selenium compounds; as a reagent for alkaloids; as oxidizing agent: L. F. Fieser, M. Fieser, Reagents for Organic Chemistry vol. 1 (New York, Wiley, 1967) p 992.
Production Methods
While selenium dioxide, SeO2, can be produced by direct reaction of the element with oxygen activated by passage through HNO3, the compound is easily made by heating selenious acid, H2SeO3. Selenium dioxide sublimes at 315–317 °C (599–603 °F), and is readily reduced by SO2 to elemental selenium.
General Description
A white or creamy-white volatile lustrous crystal or crystalline powder with a pungent sour smell. Melting point 340 deg C. Density 3.954 g / cm3 . Toxic by ingestion and inhalation.
Air & Water Reactions
In presence of water will corrode most metals [USCG, 1999]. Readily soluble in water forming selenious (selenous) acid.
Reactivity Profile
Inorganic oxidizing agents, such as Selenium dioxide , react with reducing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. Selenium dioxide reacts with water, particularly hot water, to give selenious (selenous) acid, a weak acid that is corrosive. Stable to light and heat. Rapidly absorbs dry hydrogen fluoride, hydrogen chloride, hydrogen bromide to form the corresponding selenium oxohalide. A good oxidizing agent. Reacts oxidatively with ammonia to form dinitrogen gas and selenium [Merck]. Oxidizes many organic substances.
Hazard
Toxic by inhalation, ingestion, and skin absorption.
Health Hazard
Absorption of selenium may be demonstrated by presence of the element in the urine and by a garlic-like odor of the breath. Inhalation of dust can cause bronchial spasms, symptoms of asphyxiation, and pneumonitis. Acute symptoms of ingestion include sternal pain, cough, nausea, pallor, coated tongue, gastrointestinal disorders, nervousness, and conjunctivitis. Contact with eyes causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Sublimes and forms toxic vapors when heated in fire.
Flammability and Explosibility
Non flammable
Potential Exposure
Selenium dioxide is used in the manufacture of selenium compounds, a reagent for alkaloids; an oxidizing agent; in paint and ink pigments; in metal “blueing” and etching; as a chemical catalyst; in photographic toners; in electric and photoelectric components; and others.
Shipping
UN3283Selenium compound, solid, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous material, Technical Name Required.
Purification Methods
Purify it by sublimation at 315o, or by solution in HNO3, precipitation of selenium which, after standing for several hours or boiling, is filtered off, then re-oxidised by HNO3 and cautiously evaporated to dryness below 200o. The dioxide is dissolved in H2O and again evaporated to dryness. In H2O it forms selenious acid (see selenious acid above). Its solubility in H2O is 70%w/w at 20o, and it is soluble in EtOH. [Waitkins & Clark Chem Rev 36 235 1945, Fehér in Handbook of Preparative Inorganic Chemistry (Ed Brauer) Academic Press Vol I p 421 1963.]
Incompatibilities
Selenium dioxide is and inorganic oxidizer; reacts, possibly violently, with reducing agents such as hydrides, nitrides, alkali metals, and sulfides. Contact with strong acids may cause release of toxic hydrogen selenide gas. Water solution is a medium-strong acid (selenious acid). Rapidly absorbs dry hydrogen fluoride, hydrogen bromide, hydrogen chloride, to form corresponding selenium oxohalides. Reacts with many substances producing toxic selenium vapors. Attacks many metals in presence of water.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal.
Selenium dioxide Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
Related articles
- Selenium dioxide: properties, applications and safety
- Selenium dioxide is a versatile, oxidizing compound used in various industries, but it requires careful handling due to its to....
- Dec 15,2023
- Selenium Dioxide Oxidation
- Selenium dioxide, SeO2 is an oxidizing agent generally employed in the allylic oxidation of alkenes to furnish allylic alcohol....
- Jan 28,2023
- Application and Pharmacology of Selenium dioxide
- Selenium dioxide appears as a white or creamy-white volatile lustrous crystal or crystalline powder with a pungent sour smell.....
- Jun 29,2022
View Lastest Price from Selenium dioxide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-23 | Selenium dioxide
7446-08-4
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-09-06 | Selenium(IV) oxide
7446-08-4
|
US $0.00-0.00 / kg | 1kg | 0.99 | 100tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-09-06 | Selenium dioxide
7446-08-4
|
US $0.00-0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. |
-

- Selenium dioxide
7446-08-4
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Selenium(IV) oxide
7446-08-4
- US $0.00-0.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- Selenium dioxide
7446-08-4
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.