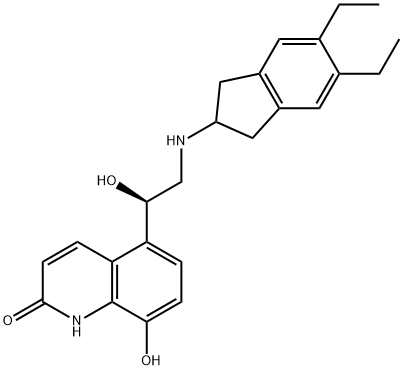
Indacaterol
- CAS No.
- 312753-06-3
- Chemical Name:
- Indacaterol
- Synonyms
- CS-272;Arcapta;Indaterol;Indacaterol;Indacaterol-d3;Indacaterol (Onbrez;Indacaterol USP/EP/BP;Indacaterol acetate salt;Indacaterol and its intermediates;Procaterol Impurity 17 ammonium salt
- CBNumber:
- CB82512068
- Molecular Formula:
- C24H28N2O3
- Molecular Weight:
- 392.49
- MDL Number:
- MFCD18782702
- MOL File:
- 312753-06-3.mol
- MSDS File:
- SDS
| Melting point | >165°C (dec.) |
|---|---|
| Boiling point | 660.3±55.0 °C(Predicted) |
| Density | 1.27 |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 8.68±0.20(Predicted) |
| form | Solid |
| color | Off-White to Light Yellow |
| InChIKey | QZZUEBNBZAPZLX-QFIPXVFZSA-N |
| SMILES | N1C2=C(C([C@@H](O)CNC3CC4=C(C3)C=C(CC)C(CC)=C4)=CC=C2O)C=CC1=O |
| FDA UNII | 8OR09251MQ |
| ATC code | R03AC18 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319-H315-H312-H332-H302-H335 | |||||||||
| Precautionary statements | P261-P271-P304+P340-P312-P264-P270-P301+P312-P330-P501-P264-P280-P302+P352-P321-P332+P313-P362-P280-P302+P352-P312-P322-P363-P501-P264-P280-P305+P351+P338-P337+P313P | |||||||||
| NFPA 704 |
|
Indacaterol price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 20070 | Indacaterol ≥98% | 312753-06-3 | 50mg | $49 | 2024-03-01 | Buy |
| Cayman Chemical | 20070 | Indacaterol ≥98% | 312753-06-3 | 100mg | $92 | 2024-03-01 | Buy |
| Cayman Chemical | 20070 | Indacaterol ≥98% | 312753-06-3 | 250mg | $216 | 2024-03-01 | Buy |
| Cayman Chemical | 20070 | Indacaterol ≥98% | 312753-06-3 | 500mg | $380 | 2024-03-01 | Buy |
| Cayman Chemical | 28886 | Indacaterol-d3 | 312753-06-3 | 1mg | $375 | 2021-12-16 | Buy |
Indacaterol Chemical Properties,Uses,Production
Description
Indacaterol is a new, ultra-long-acting, rapid onset β(2)-adrenoceptor agonist that was developed by Novartis. The drug is used in managing and controlling chronic obstructive pulmonary disease (COPD) and asthma. The European Medicines Agency (EMA) approved indacaterol as a drug in 2009 under the Onbrez trade name while in the United States the Food and Drug Administration approved it under the trade name Arcapta in 2011. The drug is manufactured as its maleate salt form. Also, indacaterol is a chiral molecule; however, only the pure R-enantiomer is distributed.
Indication
Indacaterol is used in the maintenance of airflow obstruction in individuals with COPD for the long term, including emphysema and chronic bronchitis.
Mechanism of Action
By stimulating the adrenergic beta-2-receptors in the airways’ smooth muscles, indacaterol is able to cause relaxation, thus augmenting the diameter of the airways that are normally constricted in COPD and asthma. Because of its high affinity to the lipid draft domains in the membrane of the airways, it is long acting, therefore it slowly detaches from the receptors. It is rapid acting due to its high intrinsic characteristic.
Toxicity
In case of an overdose of indacaterol, the expected signs and symptoms include excessive beta-adrenergic stimulation, hypotension, hypertension, nervousness, fatigue, hyperglycaemia, insomnia, and metabolic acidosis.
Description
Inhaled β2 adrenoceptor agonists are effective in the management of asthma and COPD, primarily through their bronchodilating properties. These drugs induce bronchodilation by causing direct relaxation of airway smooth muscle through activation of adenylate cyclase, which in turn increases intracellular cAMP levels. Indacaterol is the newest β2 agonist to reach the market. It is an ultra-long-acting agent with a duration of action suitable for once-a-day dosing. Indacaterol is supplied as an aerosol formulation of its maleate salt and is administered via a dry powder inhaler device. It is specifically approved for once-daily maintenance treatment of airflow obstruction in adult patients with COPD. In preclinical models, indacaterol is close to a full agonist at the human b2 adrenoceptor (Emax = 73 ± 1% of isoprenaline′s maximal effect, pEC50 = 8.06 ±0.02) while salmeterol displays only partial efficacy (38 ±1%). The functional selectivity profile of indacaterol over β1 human adrenoceptors is similar to that of formoterol, whereas its β3 adrenoceptor selectivity profile is similar to that of formoterol and salbutamol.
Originator
Novartis (United Kingdom)
Uses
Indacaterol is a β-adrenoreceptor agonists for treatment of asthma and bronchodilator treatment for patients with chronic obstructive pulmonary diseases.
Definition
ChEBI: A monohydroxyquinoline that consists of 5-[(1R)-2-amino-1-hydroxyethyl]-8-hydroxyquinolin-2-one having a 5,6-diethylindan-2-yl group attached to the amino function. Used as the maleate salt for treatment of chronic obstructive pulmonary di ease.
brand name
Onbrez Breezhaler
Side effects
The most commonly reported adverse events associated with indacaterol treatment were nasopharyngitis, upper respiratory tract infection, and headache and cough following inhalation. Adverse events were mild or moderate in most cases, and became less frequent with continued treatment.
Synthesis
The chemical synthesis of indacaterol begins with a-chlorination of 5-acetyl-8-benzyloxy-2-quinolone with benzyltrimethylammonium dichloro-iodate. The resultant chloroketone is reduced with borane in tetrahydrofuran in the presence of the chiral boron catalyst R-tetrahydro-1-methyl-3,3-diphenyl-1H,3H-pyrrolo[1,2c][ 1,3,2]oxazaborole to produce the corresponding chlorohydrin intermediate in high enantiomeric excess. The chlorohydrin intermediate is cyclized to the corresponding epoxide by treatment with potassium carbonate, the epoxide is condensed with 5,6-diethylindan-2-amine, and the benzyl protecting group is removed by hydrogenolysis to produce indacaterol. The 5,6-diethylindan-2-amine intermediate is derived from 1,2-diethylbenzene via Friedel-Crafts acylation with 3-chloropropionyl chloride, cyclization of the resultant 3-chloro- 1-(3,4-diethylphenyl)-1-propanone by means of concentrated sulfuric acid to 5,6-diethylindan-1-one, oximation with butyl nitrite, and reduction of the oxime to an amine via treatment with hydrogen over palladium- carbon.
Indacaterol Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| Jinan Million Pharmaceutical Co., Ltd | +86-531-68659554 +8613031714605 | info@millionpharm.com | China | 153 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Nanjing Finetech Chemical Co., Ltd. | 025-85710122 17714198479 | sales@fine-chemtech.com | CHINA | 885 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 967 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from Indacaterol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Indacaterol
312753-06-3
|
US $2.00 / kg | 1kg | 99% | 80KG/M | Jinan Million Pharmaceutical Co., Ltd | |
 |
2024-01-02 | Indacaterol
312753-06-3
|
US $70.00-700.00 / kg | 10kg | 0.99 | 20tons | Zibo Hangyu Biotechnology Development Co., Ltd | |
 |
2021-07-13 | Indacaterol
312753-06-3
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Indacaterol
312753-06-3
- US $2.00 / kg
- 99%
- Jinan Million Pharmaceutical Co., Ltd
-

- Indacaterol
312753-06-3
- US $70.00-700.00 / kg
- 0.99
- Zibo Hangyu Biotechnology Development Co., Ltd
-

- Indacaterol
312753-06-3
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
312753-06-3(Indacaterol)Related Search:
1of4