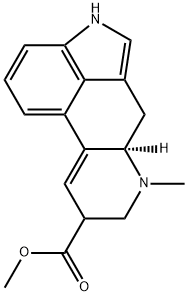
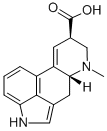
9,10-DIDEHYDRO-6-METHYL-ERGOLINE-8-CARBOXYLIC ACID
- CAS No.
- 82-58-6
- Chemical Name:
- 9,10-DIDEHYDRO-6-METHYL-ERGOLINE-8-CARBOXYLIC ACID
- Synonyms
- (6aR,9R)-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxylic acid;Lysergic;lysergicacid;Ergoline Acid;Lyserginezuur;D-Lysergic acid hydrate,95%;D-LYSERGIC ACID HYDRATE 95%;Ergometrine Maleate Impurity A;Ergometrine Maleate EP Impurity A;Methylergometrine Maleate Impurity A
- CBNumber:
- CB8261074
- Molecular Formula:
- C16H16N2O2
- Molecular Weight:
- 268.31
- MDL Number:
- MFCD00133297
- MOL File:
- 82-58-6.mol
| Melting point | >170°C (dec.) |
|---|---|
| alpha | D20 +40° (c = 0.5 in pyridine) |
| Boiling point | 411.48°C (rough estimate) |
| Density | 1.1028 (rough estimate) |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 1.6240 (estimate) |
| storage temp. | Controlled Substance, -20°C Freezer |
| pka | 3.44, 7.68(at 25℃) |
| Water Solubility | 267mg/L at 20℃ |
| LogP | -0.7 at 20℃ |
| FDA UNII | ITO20DAO7J |
9,10-DIDEHYDRO-6-METHYL-ERGOLINE-8-CARBOXYLIC ACID Chemical Properties,Uses,Production
Description
A precursor of the semisynthetic ergot derivatives, but having no biological activity itself. It is subject to controls under the Controlled Substances Act of 1970 in the United States, since it is the immediate precursor for the synthesis of LSD. Lysergic acid is obtained by hydrolysis of ergot alkaloids, either obtained from grains infected with Claviceps or, more commonly, by fermentation in submerged culture.
Chemical Properties
beige to brown powder
Uses
Labelled Lysergic acid. Lysergic acid and iso-lysergic acid are the main cleavage products formed on alkaline hydrolysis of the alkaloids which are characteristic of ergot. Potent hallucinogen; non-selective serotonin receptor agonist. Controlled substanc
Uses
Lysergic acid and iso-lysergic acid are the main cleavage products formed on alkaline hydrolysis of the alkaloids which are characteristic of ergot. Potent hallucinogen; non-selective serotonin receptor agonist. Controlled substance.
Definition
ChEBI: An ergoline alkaloid comprising 6-methylergoline having additional unsaturation at the 9,10-position and a carboxy group at the 8-position.
Synthesis Reference(s)
Journal of the American Chemical Society, 78, p. 3087, 1956 DOI: 10.1021/ja01594a039
Flammability and Explosibility
Not classified
Purification Methods
It crystallises from water as a hydrate. The methyl ester crystallises from C6H6 and has m 168o; the amide [478-94-4] has m 242o(dec) (from MeOH) and []546 +15o (c 0.5, pyridine).The (-)-hydrochloride has m 208-210o(dec, from MeOH). [Kornfeld et al. J Am Chem Soc 7 6 5256 1954,Kornfeld et al. J Am Chem Soc 78 3087 1956, Beilstein 25 III/IV 934,]
9,10-DIDEHYDRO-6-METHYL-ERGOLINE-8-CARBOXYLIC ACID Preparation Products And Raw materials
Raw materials
1of2






