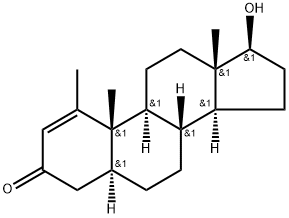
Metenolone
- CAS No.
- 153-00-4
- Chemical Name:
- Metenolone
- Synonyms
- metenolone;Methenolon;METHENOLONE;Metenolone base;Methenolone Base;Metenolone Acetat;Methenolone Tablet;Metenolone Chemical;Metenolone USP/EP/BP;Methenolone, 1.0 mg/mL
- CBNumber:
- CB8335436
- Molecular Formula:
- C20H30O2
- Molecular Weight:
- 302.46
- MDL Number:
- MFCD00273128
- MOL File:
- 153-00-4.mol
- MSDS File:
- SDS
| Melting point | 149.5-152°; mp 160-161° (Popper) |
|---|---|
| alpha | D +58.9° |
| Boiling point | 430.4±45.0 °C(Predicted) |
| Density | 1.084±0.06 g/cm3(Predicted) |
| pka | 15.08±0.70(Predicted) |
| CAS DataBase Reference | 153-00-4(CAS DataBase Reference) |
| FDA UNII | 9062ZT8Q5C |
| ATC code | A14AA04 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS07 |
|---|---|
| Signal word | Danger |
| Hazard statements | H351-H302-H362-H360 |
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501-P264-P270-P301+P312-P330-P501-P201-P260-P263-P264-P270-P308+P313 |
| DEA Controlled Substances | CSCN: 4000 CSA SCH: Schedule III NARC: No |
Metenolone price
Metenolone Chemical Properties,Uses,Production
Originator
Primobolan ,Schering ,W. Germany,1961
Uses
Methenolone is an anabolic steroid. This is a controlled substance.
Definition
ChEBI: Methenolone is a 3-hydroxy steroid. It has a role as an androgen.
Manufacturing Process
8.42 ml of methyl iodide are slowly added dropwise at room temperature with
stirring in a nitrogen atmosphere to 3.067 g of magnesium turnings and 107
ml of absolute ether. After about 30 minutes, 185 ml of absolute
tetrahydrofuran are slowly introduced and then liquid is distilled off until a
boiling point of 62°C is reached. After cooling to room temperature, 613 mg
of cuprous chloride are added and then 10 g of ?1,4,6-androstatrien-17β-ol-3-
one-17-acetate in 110 ml of tetrahydrofuran slowly introduced. After 30
minutes reaction time, the whole is cooled to 0C, the excess of Grignard
reagent decomposed with saturated ammonium chloride solution, the product
diluted with ether and the aqueous phase separated. The ethereal phase is
washed consecutively with aqueous sodium thiosulfate solution, saturated
ammonium chloride solution and water. It is dried over sodium sulfate and
evaporated to dryness under vacuum. The residue is dissolved in 40 ml of
pyridine and 20 ml of acetic anhydride and the solution kept for 16 hours at
room temperature. It is then stirred into ice water and the precipitate filtered
with suction, dried and recrystallized from isopropyl ether. 1α-Methyl-?4,6-
androstadien-17β-ol-3-one-17-acetate is obtained. MP 156°C to 157°C; [α]D25
= -33.8° (in CHCl3; c = 0.9). Yield 65-70% of the theoretical.
4.67 g of 1α-methyl-?4,6-androstadien-17β-ol-3-one-17-acetate are dissolved
in 273 ml of methanol and, after the addition of 350 mg of 10% palladium on
calcium carbonate catalyst, hydrogenated until 1 mol equivalent of hydrogen
has been taken up. After filtering off the catalyst, the solution is treated with
150 ml of 2N-hydrochloric acid and evaporated under vacuum to about 1/3 of
the volume. The whole is then diluted with water and extracted with ether.
The ethereal solution is washed with water until neutral, dried over sodium
sulfate and evaporated. The crude product is heated on a steam bath for 90
minutes in 10 ml of pyridine and 10 ml of acetic anhydride. Extraction with
ether is then carried out and the ethereal phase washed until neutral with
water. The crude crystalline 1α-methyl-?4-androsten-17β-ol-3-one-17-
acetateobtained after drying and evaporation of the solution, melts at 122°C
to 129°C. Yield 98% of the theoretical.
1α-Methyl-?4-androsten-17β-ol-3-one-17-acetate when purified by
recrystallization from isopropyl ether melts at 138°C to 139°C.
Therapeutic Function
17β-Hydroxy-1β-methyl-5α-androst-l-ene-3-one acetate
Metenolone Preparation Products And Raw materials
Raw materials
Preparation Products
Metenolone Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | admin@guyunchem.com | China | 616 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 | sales03@chemcn.cn | China | 951 | 58 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 | bruce@xrdchem.cn | CHINA | 566 | 55 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Hebei Miaobian Biotechnology Co., Ltd | +8617733850068 | CHINA | 979 | 58 | |
| Qiuxian Baitai New Material Co., LTD | +8618330912755 | sale2@hbyalin.com | China | 1677 | 58 |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | sales@sarms4muscle.com | China | 10523 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
View Lastest Price from Metenolone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-22 | Metenolone base
153-00-4
|
US $30.00 / kg | 1kg | 98% | 2000kg | hebei hongtan Biotechnology Co., Ltd | |
 |
2024-04-22 | Metenolone base
153-00-4
|
US $30.00 / kg | 1kg | 98% | 2000kg | hebei hongtan Biotechnology Co., Ltd | |
 |
2024-03-08 | Metenolone
153-00-4
|
US $10.00 / kg | 1kg | 99% | 1000kg | Nantong Guangyuan Chemicl Co,Ltd |
-

- Metenolone base
153-00-4
- US $30.00 / kg
- 98%
- hebei hongtan Biotechnology Co., Ltd
-

- Metenolone base
153-00-4
- US $30.00 / kg
- 98%
- hebei hongtan Biotechnology Co., Ltd
-

- Metenolone
153-00-4
- US $10.00 / kg
- 99%
- Nantong Guangyuan Chemicl Co,Ltd