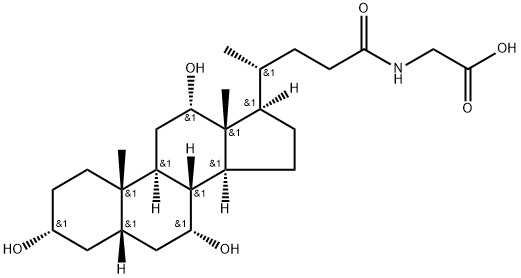
Glycocholic acid
- CAS No.
- 475-31-0
- Chemical Name:
- Glycocholic acid
- Synonyms
- GLYCITIN;cholylglycine;GLYCOCHOLIC ACID HYDRATE;N-Cholylglycine;[(3,7,12-Trihydroxy-24-oxocholan-24-yl)amino]acetic acid;GLYCOCHOLIC;N-cholyglycine;glycocholicaci;Cholylglycinate;lycocholic acid
- CBNumber:
- CB8378590
- Molecular Formula:
- C26H43NO6
- Molecular Weight:
- 465.63
- MDL Number:
- MFCD00065902
- MOL File:
- 475-31-0.mol
| Melting point | 128°C |
|---|---|
| Boiling point | 568.76°C (rough estimate) |
| Density | 1.1336 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Refrigerator |
| solubility | methanol: 0.1 g/mL, clear, colorless |
| form | Solid |
| pka | 4.4(at 25℃) |
| color | White to Off-White |
| Water Solubility | 329.9mg/L(20 ºC) |
| Merck | 13,4507 |
| Stability | Hygroscopic |
| InChIKey | RFDAIACWWDREDC-NSPZZGDONA-N |
| LogP | 1.650 |
| Substances Added to Food (formerly EAFUS) | GLYCOCHOLIC ACID |
| SCOGS (Select Committee on GRAS Substances) | Glycocholic acid |
| CAS DataBase Reference | 475-31-0(CAS DataBase Reference) |
| FDA UNII | G59NX3I3RT |
| NIST Chemistry Reference | Glycocholic acid(475-31-0) |
Glycocholic acid price More Price(33)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 20276 | Glycocholic Acid ≥95% | 475-31-0 | 1g | $48 | 2024-03-01 | Buy |
| Cayman Chemical | 20276 | Glycocholic Acid ≥95% | 475-31-0 | 5g | $152 | 2024-03-01 | Buy |
| Cayman Chemical | 20276 | Glycocholic Acid ≥95% | 475-31-0 | 10g | $278 | 2024-03-01 | Buy |
| Usbiological | 278290 | Glycocholic acid | 475-31-0 | 1g | $336 | 2021-12-16 | Buy |
| TRC | G641370 | GlycocholicAcid | 475-31-0 | 5g | $185 | 2021-12-16 | Buy |
Glycocholic acid Chemical Properties,Uses,Production
Description
N-Cholylglycine. Bile salt, conjugate of cholate and glycine, usually as the sodium salt. It acts as a detergent to solubilize fats for absorption and is itself absorbed. It is used as a cholagogue and choleretic.
Description
Glycocholic acid is a glycine-conjugated form of the primary bile acid cholic acid and has roles in the emulsification of fats. It reduces expression of the gene encoding the farnesoid X receptor (FXR) and increases expression of the genes encoding the bile acid receptors TGR5 and S1PR2 in SNU-245 cells when used at a concentration of 1.6 μmol/ml. Glycocholic acid (250 μM) increases the intracellular accumulation and cytotoxicity of epirubicin in Caco-2 cells, as well as decreases expression of the genes encoding multidrug resistance protein 1 (MDR1), MDR-associated protein 1 (MRP1), and MRP2 when used alone or in combination with epirubicin. It increases absorption of epirubicin into everted sacs of rat ileum and jejunum when used at a concentration of 250 μM. The bile acid composition ratio of glycocholic acid is elevated in bile of patients with cholangiocarcinoma compared with patients with pancreatic cancer or benign biliary diseases. Serum levels of glycocholic acid are elevated in patients with hepatocellular carcinoma compared with healthy individuals.
Chemical Properties
Off-White Solid
Uses
Labelled Glycocholic Acid. The product of conjugation of cholic acid with glycine; chief ingredient of the bile of herbivorous animals. In the weakly alkaline bile fluid glycocholic acid exists as the sodium salt.
Definition
ChEBI: A bile acid glycine conjugate having cholic acid as the bile acid component.
Purification Methods
Glycocholic acid crystallises from hot water as the sesquihydrate. Dry it at 110o in vacuo. An analytical sample is prepared by suspending the acid (4g) in H2O (400mL) at ~20o, heating to boiling with slow stirring, filtering hot and allowing to cool to ~20o. The acid is filtered off, washed with H2O, dried in air, recrystallised from 5% aqueous EtOH, washed well and dried over P2O5 in a moderate vacuum to constant weight. Recrystallisation from EtOH/EtOAc, and drying, gave the anhydrous acid. [Cortese & Bauman J Am Chem Soc 57 1393 1935, Bergstrom & Norman Acta Chem Scand 7 1126 1953, Beilstein 10 IV 2077.]
Glycocholic acid Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Sense Chemicals (Shanghai) Co., Ltd. | +86-21-52751036; +8613818977689 | david.dai@sensechemicals.com | China | 224 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7786 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@airuikechemical.com | China | 994 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from Glycocholic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-08 | Glycocholic acid
475-31-0
|
US $0.00-0.00 / Kg | 1Kg | 99.9% | 200tons | airuikechemical co., ltd. | |
 |
2024-04-05 | Glycocholic acid
475-31-0
|
US $34.00-1.20 / kg | 1kg | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2024-03-16 | Glycocholic acid
475-31-0
|
US $0.00 / KG | 100g | 98%+ | 100kg | WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD |
-

- Glycocholic acid
475-31-0
- US $0.00-0.00 / Kg
- 99.9%
- airuikechemical co., ltd.
-

- Glycocholic acid
475-31-0
- US $34.00-1.20 / kg
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Glycocholic acid
475-31-0
- US $0.00 / KG
- 98%+
- WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD