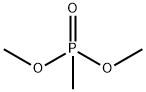
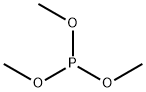
Dimethyl methylphosphonate
- CAS No.
- 756-79-6
- Chemical Name:
- Dimethyl methylphosphonate
- Synonyms
- DMMP;Fyrol dmmp;DIMETHYL METHANEPHOSPHONATE;Metaran;pyroldmmp;NSC 62240;fyroldmmp;NCI-C54762;Pyrol DMMP;Reoflam DMMP
- CBNumber:
- CB8418753
- Molecular Formula:
- C3H9O3P
- Molecular Weight:
- 124.08
- MDL Number:
- MFCD00008349
- MOL File:
- 756-79-6.mol
- MSDS File:
- SDS
| Melting point | <50° |
|---|---|
| Boiling point | 181 °C(lit.) |
| Density | 1.145 g/mL at 25 °C(lit.) |
| vapor pressure | <0.1 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 156 °F |
| solubility | Chloroform, Methanol (Slightly, Heated) |
| pka | pKa (20°) in water: 2.37 |
| form | Liquid |
| color | Clear colorless |
| Specific Gravity | 1.16 |
| Water Solubility | >=10 g/100 mL at 21 ºC |
| Merck | 14,3391 |
| BRN | 878263 |
| Stability | Stability Combustible. Incompatible with strong oxidizing agents, strong bases. May soften some rubbers or plastics. Hydrolyzes slowly in contact with water. |
| LogP | -0.61 |
| Dissociation constant | 2.37 at 20℃ |
| CAS DataBase Reference | 756-79-6(CAS DataBase Reference) |
| FDA UNII | 20Z996230U |
| NIST Chemistry Reference | Phosphonic acid, methyl-, dimethyl ester(756-79-6) |
| EPA Substance Registry System | Dimethyl methylphosphonate (756-79-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H319-H340-H361f | |||||||||
| Precautionary statements | P201-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | T,Xi | |||||||||
| Risk Statements | 46-36-62 | |||||||||
| Safety Statements | 53-26-45 | |||||||||
| RIDADR | 2810 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | SZ9120000 | |||||||||
| Hazard Note | Irritant/Toxic | |||||||||
| TSCA | Yes | |||||||||
| Toxicity | LD50 in rats, mice (mg/kg): 10190, >6810 orally (Rowland) | |||||||||
| NFPA 704 |
|
Dimethyl methylphosphonate Chemical Properties,Uses,Production
Chemical Properties
colourless liquid
Uses
As a flame retardant, a preignition additive for gasoline, an antifoam agent, a plasticizer and stabilizer, a textile conditioner, and an antistatic agent; used experimentally to mimic the physical and spectroscopic (but not biological) properties of anticholinesterase agents
Uses
Dimethyl methylphosphonate is used as a catalyst and a reagent in organic synthesis for the conversion of esters to ketophosphonates. It is an additive for the synthesis of unsaturated polyester resin which has flame retardant high phosphorous and UV-cured epoxy acrylate. It finds application as hydraulic fluids as well.
Uses
NMR probe for cell volume. Flame retardant. Simulant for nerve agents.
Synthesis Reference(s)
Synthetic Communications, 20, p. 239, 1990 DOI: 10.1080/00397919008052289
General Description
Clear colorless liquid with a pleasant odor.
Air & Water Reactions
Highly flammable. Water soluble. Hydrolyzes slowly upon contact with water.
Reactivity Profile
Dimethyl methylphosphonate is incompatible with strong oxidizing agents and strong bases. Dimethyl methylphosphonate reacts with organic halides at 302-392° F. When heated to temperatures greater than 302° F, Dimethyl methylphosphonate will act as an alkylating agent with basic nitrogen compounds and phenols. Dimethyl methylphosphonate reacts with enol lactones. Dimethyl methylphosphonate has plasticizing properties and may soften or deteriorate some plastics and elastomers (particularly vinyl-based resins, neoprene and natural rubbers) upon contact.
Health Hazard
Dimethyl methylphosphonate (DMMP) administered to male rats is a reproductive toxicant and carcinogen. Effects in humans are unknown.
Fire Hazard
Dimethyl methylphosphonate is combustible.
Safety Profile
Moderately toxic by intravenous route. Experimental reproductive effects. Questionable carcinogen with experimental carcinogenic data. Mutation data reported. An experimental nerve gas stimulant. A flame retardant. When heated to decomposition it emits toxic fumes of POx
Dimethyl methylphosphonate Preparation Products And Raw materials
756-79-6(Dimethyl methylphosphonate)Related Search:
1of4