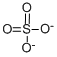
SULFATE STANDARD
- CAS No.
- 14808-79-8
- Chemical Name:
- SULFATE STANDARD
- Synonyms
- SULPHATE;SULFATE ION;Sulfate ions;Sulfate anion;Sulfate dianion;SULFATE STANDARD;sulphate-sulfate;Sulfate (ion 2-);Sulfate anion(2-);SULFATE IC STANDARD
- CBNumber:
- CB8466491
- Molecular Formula:
- O4S-2
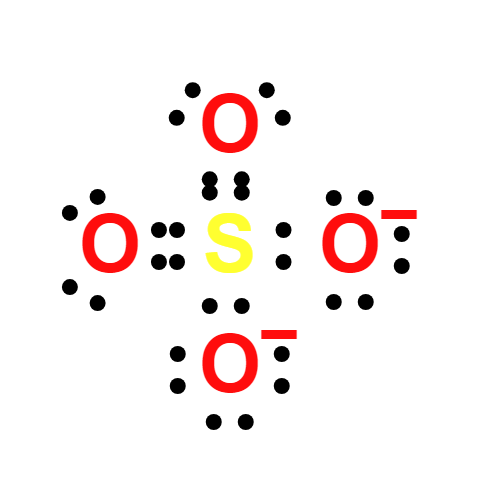
Lewis structure
- Molecular Weight:
- 96.06
- MDL Number:
- MFCD00145343
- MOL File:
- 14808-79-8.mol
- MSDS File:
- SDS
| storage temp. | 2-8°C |
|---|---|
| form | Liquid |
| color | Clear colorless |
| BRN | 3648446 |
| CAS DataBase Reference | 14808-79-8(CAS DataBase Reference) |
| FDA UNII | 7IS9N8KPMG |
| EPA Substance Registry System | Sulfate (14808-79-8) |
SULFATE STANDARD price More Price(3)
SULFATE STANDARD Chemical Properties,Uses,Production
Description
The sulfate anion (SO42−) is the stable, oxidized form of sulfur. Sulfate minerals are widely distributed in nature, and most sulfate compounds are readily soluble in water. All sulfate salts are very soluble except for calcium and silver sulfates, which are moderately soluble, and barium, mercury, lead, and strontium sulfates, which are insoluble.
It is estimated that about one-half of the river sulfate load arises from mineral weathering and volcanism, and the other half from biochemical and anthropogenic sources. Industrial discharges are another significant source of sulfates. Mine and tailings drainage, smelter emissions, agricultural runoff from fertilized lands, pulp and paper mills, textile mills, tanneries, sulfuric acid production, and metalworking industries are all sources of sulfate-polluted water. Aluminum sulfate (alum) is used as a sedimentation agent for treating drinking water. Copper sulfate is used for controlling algae in raw and public water supplies.
Definition
ChEBI: A sulfur oxoanion obtained by deprotonation of both OH groups of sulfuric acid.
Health Hazard
The sulfate anion is generally considered nontoxic to animal, aquatic, and plant life. It is an important source of sulfur, an essential nutrient for plants and animals. Sulfates are used as additives in the food industry, and the average daily intake of sulfate from drinking water, air, and food is approximately 500 mg. As examples, some measured sulfate concentrations in beverages are 100–500 mg/L in drinking water, 500 mg/L in coconut milk, 260 mg/L in beer (bitter), 250 mg/L in tomato juice, and 300 mg/L in red wine (FNB 2004). Available data suggest that people acclimate rapidly to the presence of sulfates in their drinking water.
No upper limit likely to cause detrimental human health effects has been determined for sulfate in drinking water. However, concentrations of 500–750 mg/L may cause a temporary mild laxative effect, although doses of several thousand milligrams per liter generally do not cause any long-term ill effects. Because of the laxative effects resulting from ingestion of drinking water containing high sulfate levels, the EPA recommends that health authorities be notified of sources of drinking water that contain sulfate concentrations in excess of 500 mg/L.
The presence of sulfate can adversely affect the taste of drinking water, imparting a bitter taste. The lowest taste threshold concentration for sulfate is approximately 250 mg/L as sodium salt, but higher as calcium or magnesium salts (up to 1000 mg/L).
Environmental Fate
Nearly all natural surface waters and shallow groundwaters contain sulfate anions. Sulfate is commonly found as a prominent component of unpolluted waters and is included among the six major surface and shallow groundwater ions (Na+ , Ca+ , Mg+Cl− , (HCO3)2− , and (SO4)2−), second to bicarbonate as the most abundant anion in most freshwaters. Sulfur is an essential plant and animal nutrient, and sulfate is the most common inorganic form of sulfur in aerobic environments. Sulfate water concentrations that are too low have a detrimental effect on both land and aquatic plant growth.
Sulfate is redox sensitive and is bacterially reduced to sulfide ion under anaerobic conditions. Sulfide may be released to the atmosphere as H2S gas or precipitated as insoluble metal sulfides. Oxidation of sulfides returns sulfur to the sulfate form.
Sulfates may be leached from most sedimentary rocks, including shales, with the most appreciable contributions from such sulfate deposits as gypsum (CaSO4·2H2O) and anhydrite (CaSO4 ). The oxidation of sulfur-bearing organic materials can con- tribute sulfates to waters.
SULFATE STANDARD Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
SULFATE STANDARD Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Dideu Medichem Co. Ltd | 18192627656 | 1012@dideu.com | China | 3657 | 58 |
| VladaChem GmbH | +49-7246-3082843 | info@vladachem.de | Germany | 1860 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Shanghai BeiZhuo Biotech Co., Ltd. | 021-61119791,13386096464 | bzswkf@foxmail.com | China | 3924 | 50 |
| Chengdu HuaXia Chemical Reagent Co. Ltd | 400-1166-196 13458535857 | cdhxsj@163.com | China | 13358 | 58 |
| VWR(Shanghai) Co., Ltd | 400-821-8006 | info_china@vwr.com | China | 3041 | 75 |
| Zhongxiang Yaowei Biological Technology Co., Ltd. | 15337241005 13260682861 | w13260682861@qq.com | China | 2437 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | 029-61856359 18690052321 | 1027@dideu.com | China | 9927 | 58 |
Related articles
- The Lewis structure of Sulfate Standard
- Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid, and many are prepared from that acid.
- Nov 29,2023
View Lastest Price from SULFATE STANDARD manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2020-03-16 | SULFATE STANDARD
14808-79-8
|
US $1.00-0.00 / KG | 1KG | 99% | 20000KG | Shaanxi Dideu Medichem Co. Ltd |
-

- SULFATE STANDARD
14808-79-8
- US $1.00-0.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd
14808-79-8(SULFATE STANDARD)Related Search:
1of4