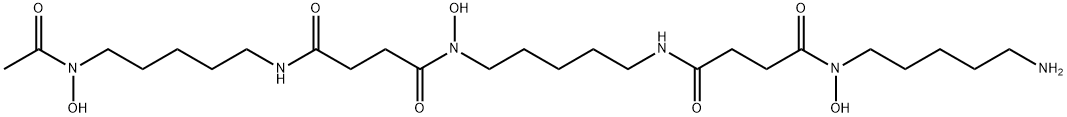
Deferoxamine
- CAS No.
- 70-51-9
- Chemical Name:
- Deferoxamine
- Synonyms
- DESFEROXAMINE;dfoa;taone;entaone;ba33112;desferin;desferex;desferan;ba-29837;ba-33112
- CBNumber:
- CB8506290
- Molecular Formula:
- C25H48N6O8
- Molecular Weight:
- 560.69
- MDL Number:
- MFCD00242585
- MOL File:
- 70-51-9.mol
| Melting point | 139°C |
|---|---|
| Boiling point | 627.9°C (rough estimate) |
| Density | 1.2216 (rough estimate) |
| refractive index | 1.5540 (estimate) |
| pka | 9.08±0.50(Predicted) |
| NCI Dictionary of Cancer Terms | deferoxamine |
| FDA UNII | J06Y7MXW4D |
| ATC code | V03AC01 |
| EPA Substance Registry System | Deferoxamine (70-51-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H317 |
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501 |
| Toxicity | LD50 oral in mouse: 1340mg/kg |
Deferoxamine price
Deferoxamine Chemical Properties,Uses,Production
Description
Deferoxamine was introduced in the 1960s for chelation of iron. It is synthesized by removing a central iron molecule from ferrioxamine B, a compound obtained from the microorganism Streptomyces pilosus. Deferoxamine binds to iron from ferritin and forms ferrioxamine, a very stable and water-soluble chelate with a characteristic reddish color.
Originator
Deferoxamine,Novartis,Germany
Uses
Chelating agent (iron).
Uses
Deferoxamine is used for the treatment of both acute iron intoxication and chronic iron overload due to transfusiondependent anemias. It has also been used in trials for malaria treatment and for aluminum chelation in hemodialysis patients. Studies of a rat model of intracerebral hemorrhage have noted that deferoxamine treatment reduced oxidative stress from iron release, indicating a possible role in preventing damage associated with hemorrhagic strokes.
Definition
ChEBI: Desferrioxamine B is an acyclic desferrioxamine that is butanedioic acid in which one of the carboxy groups undergoes formal condensation with the primary amino group of N-(5-aminopentyl)-N-hydroxyacetamide and the second carboxy group undergoes formal condensation with the hydroxyamino group of N(1)-(5-aminopentyl)-N(1)-hydroxy-N(4)-[5-(hydroxyamino)pentyl]butanediamide. It is a siderophore native to Streptomyces pilosus biosynthesised by the DesABCD enzyme cluster as a high affinity Fe(III) chelator. It has a role as an iron chelator, a siderophore, a ferroptosis inhibitor and a bacterial metabolite. It is a conjugate acid of a desferrioxamine B(3-).
Manufacturing Process
O-Benzylhydroxylamine hydrochloride (4.7 g, 29.7 mmol) was mixed with 5
ml of water and 11 ml of methanol at 0°C and the pH adjusted to 4.7 using 6
N KOH. The aldehyde, 4-cyanobutanal (2.6 mL, 27 mmol) was added to the
hydroxylamine and the mixture allowed to warm to room temperature. The pH
was maintained by addition of further 6 N KOH. After 1 h, the reaction was
cooled to 0°C, and sodium cyanoborohydride (1.26 g, 20 mmol) was added.
The pH was adjusted to 3 and maintained by addition of saturated HCl in
methanol. When the pH stabilized, the reaction was warmed to room temperature and stirred for 3 h at a PH of 3. The reaction mixture was then
poured into ether and made basic with 6 N KOH. The aqueous layer was
extracted with ether (3x50 mL). The extracts were combined, washed with
brine, and dried over magnesium sulfate. The solvents were removed and the
resulting liquid distilled at 150°-151°C (0.6 mm) to give 4.65 g (84% of Obenzyl-N-(4-cyanobutyl)hydroxylamine. 2.8 g (13.7 mmol) of the above
prepared hydroxylamine in 23 ml of pyridine and 2.1 g (20.8 mmol) of
succcinic anhydride, initially heated at 100°C for 1.5 h then allowed to cool to
room temperature and stirred overnight. The pyridine was removed in vacuum
and the residue was dissolved in a minimal amount of chloroform, and the
residue was dissolved in ether, which was extracted three times with 20%
potassium bicarbonate (3x50 mL). The aqueous solutions were combined,
acidified, extracted with ether, dried, filtered and evaporated; the residue was
then chromotagraphed on silica gel to give 4.12 g (98%) of N-(4-cyanobutylN-(benzyloxy)succinamic acid.
2.6 g (12.75 mmol) of O-benzyl-N-(4-cyanobutyl)hydroxylamine, 17.24 mL of
pyridine and 17.2 mL of acetic anhydride were stirred under argon at room
temperature for 24 h. Then the excess pyridine and acetic acid anhydride
were removed by vacuum. The resulting oil was taken up in chloroform, which
was extracted with 1 N HCl (2x50mL), washed with sodium bicarbonate and
brine, dried, over sodium sulfate, filtered and evaporated to give 3.4 g
(100%) of N-(4-cyanobutyl)-N-(benzyloxy)acetamide as a light oil. 1.4 g (5.7
mmol) of this product, 2.6 g Raney nickel, 15 ml of ammonia saturated
methanol and 4 ml of saturated ammonium hydroxide were cooled in a ice
bath and anhydrous ammonia was allowed to bubble through the solution for
10 min. The bottle was pressurized to 50 psi with hydrogen and shook for 3 h.
Then the catalyst was filtered and the solvents evaporated. The crude material
was chromatografed on silica gel to gave a 1.25 g (88%) of N-(5-
aminopentyl)-N-(benzyloxy)acetamide.
1 g (4 mmol), of the above acetamide, 1.46 g (4.79 mmol) of N-(4-
cyanobutyl-N-(benzyloxy)succinamic acid, 1.24 g (6 mmol) of DCC and 70 mg
of DMAP was cooled to 0°C for 0.55 h in 28 mL of chloroform. The mixture
was allowed to warm to room temperature and stirred 24 h. Then it was again
cooled to 0°C, filtered and chromatografed to yield 2.1 g (98%) of N-(4-
cyanobutyl)-3-[{5-N-benzyloxy)acetamido)pentyl}carbomoyl]-Obenzylpropionohydroxamic acid. This product (1 g) was hydrogeneted by
analogue with N-(4-cyanobutyl)-N-(benzyloxy)acetamide using Nickel Raney
as catalyst to give 1 g (88%) N-(5-aminopentyl)-3-[{5-(N -
benzyloxyacetamido)pentyl}carbomoyl]-O-benzylpropionohydroxamic acid,
which produced by the reaction with DCC described above 0.78 g (88%) of N-
[5-[3-[{4-cyanobutyl)(benzyloxy)-carbomoyl]propionaminoamido]pentyl}-3-
[{5-(N-bebzyloxyacetamido)pentyl]-carbomoyl]-O-benzylpropionohydroxamic
acid. The purity of all products confirmed with1H-NMR and elemental
analyses. The last compound (0.165 g, 0.2 mmol) was reduced in methanol,
2.7 mL of 0.1 N HCl and 0.27 g of 10% Pd on C. The hydrogenation was
carried out at one atmosphere of hydrogene for 7.5 hrs. The solution was
filtered, the solvents were removed and the residue was washed with cold
methanol, and then chloroform to give 0.1 g (84%) of product. This material
had melting point 167°-168°C [Prelog, supra] and was identical to an
authentic sample by 300 MHz NMR [sample of deferrioxamine B supplied by
dr. Heirich H. Peter at Ciba-Geigy, Basel, Switzerland].
brand name
Desferal (Novartis).
Therapeutic Function
Pharmaceutic aid (chelating agent)
Environmental Fate
Localized infusion or injection site reactions may occur with deferoxamine administration, such as pain, urticaria and flushing of the skin. Hypersensitivity reactions have been documented with both acute and chronic administration of deferoxamine. Some of the more serious side effects include infusion rate-related hypotension, renal insufficiency, neurotoxicity, growth retardation, pulmonary toxicity, and infections. Deferoxamine may induce venous dilation when given at doses greater than 15 mg kg-1 h-1 leading to poor venous return, depressed cardiac output, and eventually hypotension. Increased levels of histamine have been noted during hypotensive episodes, although pretreatment with antihistamines has not been shown to stop the reaction. An acute decrease in glomerular filtration rate and renal plasma flow secondary to hypotension is the possible mechanism underlying the nephrotoxicity induced by deferoxamine. Depletion of iron, translocation of copper, and chelation of other trace elements including zinc may interfere with critical iron-dependent enzymes, causing oxidative damage within various tissues. These are possible mechanisms thought to be responsible for deferoxamineinduced neurotoxicity, growth retardation, and pulmonary toxicity. In vitro studies have shown that deferoxamine inhibits the synthesis of prostaglandin, hemoglobin, ferritin, collagen, and DNA. The iron–deferoxamine complex, ferrioxamine, is a growth factor for many bacteria and fungi. Deferoxamine has been associated with Yersinia enterocolitica overgrowth and fatal cases of mucormycosis with prolonged therapy.
Deferoxamine Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9553 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29474 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 | peter68@ptchemgroup.com | China | 35453 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8474 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131981 | 58 |
Related articles
- Side effects of Deferoxamine
- Deferoxamine was introduced in the 1960s for chelation of iron. It is synthesized by removing a central iron molecule from fer....
- Jan 17,2022
View Lastest Price from Deferoxamine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-17 | Deferoxamine
70-51-9
|
US $1.10 / g | 1g | 99.0% min | 100 tons min | Shaanxi Dideu Medichem Co. Ltd | |
 |
2024-03-20 | Deferoxamine
70-51-9
|
US $8.00 / mg | 10mg | 98% | 50g | Wuhan Topule Biopharmaceutical Co., Ltd | |
 |
2021-08-14 | Deferoxamine USP/EP/BP
70-51-9
|
US $1.10 / g | 1g | 0.999 | 100 Tons min | Dideu Industries Group Limited |
-

- Deferoxamine
70-51-9
- US $1.10 / g
- 99.0% min
- Shaanxi Dideu Medichem Co. Ltd
-

- Deferoxamine
70-51-9
- US $8.00 / mg
- 98%
- Wuhan Topule Biopharmaceutical Co., Ltd
-

- Deferoxamine USP/EP/BP
70-51-9
- US $1.10 / g
- 0.999
- Dideu Industries Group Limited
70-51-9(Deferoxamine)Related Search:
1of4