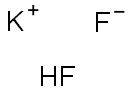Potassium hydrogen fluoride
- CAS No.
- 7789-29-9
- Chemical Name:
- Potassium hydrogen fluoride
- Synonyms
- POTASSIUM BIFLUORIDE;POTASSIUM HYDROGEN DIFLUORIDE;K(HF2);NA 1811;en difL;Potassium hydrog;Potassium hydrogen f;Kaliumhydrogenfluorid;bifluoruredepotassium;potassiumacidfluoride
- CBNumber:
- CB8854344
- Molecular Formula:
- F2HK
- Molecular Weight:
- 78.1
- MDL Number:
- MFCD00011403
- MOL File:
- 7789-29-9.mol
- MSDS File:
- SDS
| Melting point | 239 °C(lit.) |
|---|---|
| Density | 2.37 g/mL at 25 °C(lit.) |
| solubility | insoluble in ethanol |
| form | Crystals or Crystalline Powder |
| Specific Gravity | 2.37 |
| color | White to light gray |
| Water Solubility | 39 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,7610 |
| Exposure limits |
ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3 |
| CAS DataBase Reference | 7789-29-9(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 880X05W53M |
| NIST Chemistry Reference | Potassium hydrogen fluoride(7789-29-9) |
| EPA Substance Registry System | Potassium bifluoride (7789-29-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H314 | |||||||||
| Precautionary statements | P260-P280-P301+P310+P330-P301+P330+P331-P303+P361+P353-P305+P351+P338+P310 | |||||||||
| Hazard Codes | T,C | |||||||||
| Risk Statements | 25-34 | |||||||||
| Safety Statements | 26-36/37/39-45-37-22 | |||||||||
| RIDADR | UN 3421 8/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | TS6650000 | |||||||||
| F | 3 | |||||||||
| Hazard Note | Corrosive | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28261990 | |||||||||
| NFPA 704 |
|
Potassium hydrogen fluoride price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 239283 | Potassium hydrogenfluoride 99% | 7789-29-9 | 25g | $56.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 239283 | Potassium hydrogenfluoride 99% | 7789-29-9 | 2.5kg | $313 | 2024-03-01 | Buy |
| Sigma-Aldrich | 60344 | Potassium hydrogen difluoride purum p.a., ≥99.0% (F) | 7789-29-9 | 250G | $71.6 | 2023-06-20 | Buy |
| Sigma-Aldrich | 60344 | Potassium hydrogen difluoride purum p.a., ≥99.0% (F) | 7789-29-9 | 1KG | $173 | 2023-06-20 | Buy |
| Alfa Aesar | 011557 | Potassium hydrogen fluoride, 99+% (metals basis) | 7789-29-9 | 500g | $64.2 | 2023-06-20 | Buy |
Potassium hydrogen fluoride Chemical Properties,Uses,Production
Description
Potassium hydrogen fluoride is used as an etchant to etch glass surface and it is used in the cleaning products. It is used as a fluorinating agent for fluorinated organic compounds, which could be applied as finishing agent for fabrics, components of extinguishing agents, electroplating bathes, lubricating oils, oxygen carriers in blood substitutes. For example, potassium hydrogen fluoride is used as fluorinating agent for ring opening of epoxides and cyclopropanes and in the synthesis of trifluoroborates from various organoborons. It may also serve as a fluoride ion source for the nucleophilic ring-opening reaction of epoxide.
References
[1] Gary A. Molander, David J. Cooper and Stephen W. Wright, Potassium Hydrogen Fluoride, e-EROS Encyclopedia of Reagents for Organic Synthesis, 2001
[2] Bernd Basner, Houben-Weyl Methods of Organic Chemistry Vol. E 10b/1, 4th Edition, 1999
[3] Francis I. Onuska and Francis W. Karasek, Open Tubular Column Gas Chromatography in Environmental Sciences, 1984
[4] Michael Howe, Wheel cleaning composition containing acid fluoride salts, Patent “US 5556833 A”, 1996
[5] Grzegorz Lewandowski, Egbert Meissner and Eugeniusz Milchert, Special applications of fluorinated organic compounds, Journal of Hazardous Materials, 2006, vol. 136, 385-391
Chemical Properties
WHITE TO LIGHT GREY CRYSTALS OR CRYSTALLINE POWDER
Uses
Potassium hydrogen fluoride is widely used as an etchant in glass and cleaning products. It finds an important application in the synthesis of special optical glass viz. crown and crown flint glass and as a catalyst for polymerization. It is involved in the manufacturing of wood preservatives, soldering agents and brazing. It is also used in the manufacturing of organic and inorganic fluorine compounds.
Uses
In the preparation of pure potassium fluoride; as an electrolyte in the manufacture of fluorine; frosting glass; treating coal to prevent slag formation; flux for silver solders; catalyst in the alkylation of benzene with olefins.
General Description
Colorless crystals, corrosive to tissues, etches glass.
Air & Water Reactions
Water soluble giving strongly acidic solutions.
Reactivity Profile
Acidic salts, such as Potassium hydrogen fluoride, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Hazard
Corrosive to tissue.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
A poison. Very corrosive and reactive. A corrosive irritant to the eyes, skin, and mucous membranes. When heated to decomposition it emits toxic fumes of F and K2O. See also FLUORIDES and HYDROFLUORIC ACID.
Purification Methods
It crystallises from water. It is very soluble in hot H2O and 41% at 21o. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 237 1963.]
Potassium hydrogen fluoride Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
View Lastest Price from Potassium hydrogen fluoride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-10-11 | Potassium hydrogen fluoride
7789-29-9
|
US $30.00-9.00 / kg | 1kg | 0.99 | 10 tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-09-20 | Potassium hydrogen fluoride
7789-29-9
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-09-06 | Potassium hydrogen fluoride
7789-29-9
|
US $0.00-0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. |
-

- Potassium hydrogen fluoride
7789-29-9
- US $30.00-9.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- Potassium hydrogen fluoride
7789-29-9
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Potassium hydrogen fluoride
7789-29-9
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.