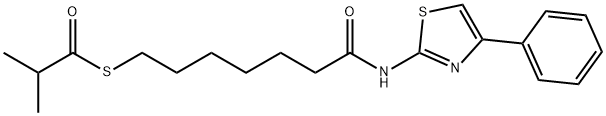
PTACH
- CAS No.
- 848354-66-5
- Chemical Name:
- PTACH
- Synonyms
- NCH 51;NCH51, >98%;PTACH?, >98%;PTACH (NCH-51);HDAC Inhibitor XXII, NCH51;HDAC Inhibitor XXII, NCH51 - CAS 848354-66-5 - Calbiochem;Cpd 51, S-[6-(4-Phenyl-2-thiazolylcarbamoyl)hexyl] thioisobutyrate;S-(7-Oxo-7-((4-phenylthiazol-2-yl)amino)-heptyl) 2-methylpropanethioate;S-[7-oxo-7-[(4-phenyl-1,3-thiazol-2-yl)amino]heptyl] 2-methylpropanethioate;2-MethylpropanethioicacidS-[7-oxo-7-[(4-phenyl-2-thiazolyl)amino]heptyl]ester
- CBNumber:
- CB8972794
- Molecular Formula:
- C20H26N2O2S2
- Molecular Weight:
- 390.56
- MDL Number:
- MFCD08705329
- MOL File:
- 848354-66-5.mol
PTACH price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 382185 | HDAC Inhibitor XXII, NCH51 | 848354-66-5 | 2mg | $140 | 2023-01-07 | Buy |
| Sigma-Aldrich | P5874 | PTACH ≥98% (HPLC), solid | 848354-66-5 | 2mg | $286 | 2022-05-15 | Buy |
| Cayman Chemical | 21234 | NCH-51 ≥98% | 848354-66-5 | 1 mg | $26 | 2024-03-01 | Buy |
| Cayman Chemical | 21234 | NCH-51 ≥98% | 848354-66-5 | 5mg | $96 | 2024-03-01 | Buy |
| Cayman Chemical | 21234 | NCH-51 ≥98% | 848354-66-5 | 10mg | $153 | 2024-03-01 | Buy |
PTACH Chemical Properties,Uses,Production
Uses
NCH 51 is a Histone deacetylase inhibitor.
Definition
ChEBI: 2-methylpropanethioic acid S-[7-oxo-7-[(4-phenyl-2-thiazolyl)amino]heptyl] ester is an aromatic amide.
General Description
A cell-permeable S-isobutyryl prodrug that is processed intracellularly to form the potent HDAC inhibitor NCH-31 (IC50 = 48 and 170 nM, respectively, using partially purified HDAC1 or HeLa nuclear extract) that is predicted to exhibit a similar HADC binding mode as that of SAHA with its sulfhydryl replacing SAHA′s hydroxamate as the active-site zinc-targeting group. NCH-51 is shown to exhibit comparable antiproliferative (mean IC50 = 3.8 μM vs. 3.7 μM for SAHA; 48 h treatment) and apoptotic activity as SAHA against various cancer cell lines, but not PBMCs from 4 healthy individuals (IC50 >30 μM with either drug), and the antioxidant N-Acetyl-L-Cysteine (NAC; Cat. No. 106425) at 2 mM is reported to abolish the cell-growth inhibition caused by either 3 μM NCH-51 or SAHA in the Multiple Myeloma U266 cultures. Half-life in human plasma at 37 °C = 24 h.
Biological Activity
Histone deacetylase (HDAC) inhibitor. Inhibits growth of various cancer cells in vitro (EC 50 = 1.1-9.1 μ M).
Biochem/physiol Actions
HDAC inhibitor; more potent than the majority of HDAC inhibitors except for SAHA (gold standard).
References
[1]. suzuki t, hisakawa s, itoh y, et al. identification of a potent and stable antiproliferative agent by the prodrug formation of a thiolate histone deacetylase inhibitor. bioorg med chem lett, 2007, 17(6): 1558-1561.
[2]. suzuki t, nagano y, kouketsu a, et al. novel inhibitors of human histone deacetylases: design, synthesis, enzyme inhibition, and cancer cell growth inhibition of saha-based non-hydroxamates. j med chem, 2005, 48(4): 1019-1032.
[3]. sanda t, okamoto t, uchida y, et al. proteome analyses of the growth inhibitory effects of nch-51, a novel histone deacetylase inhibitor, on lymphoid malignant cells. leukemia, 2007, 21(11): 2344-2353.
[4]. victoriano af, imai k, togami h, et al. novel histone deacetylase inhibitor nch-51 activates latent hiv-1 gene expression. febs lett, 2011, 585(7): 1103-1111.
PTACH Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | sales@sarms4muscle.com | China | 10523 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6393 | 58 |
| Nanjing Doge Biomedical Technology Co., Ltd | +86-25-58227606 +86-15305155328 | sales@dogechemical.com | China | 4128 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 | support@targetmol.com | United States | 19973 | 58 |
| ShenZhen Trendseen Biological Technology Co.,Ltd. | 13417589054 | trendseenbio@gmail.com | China | 11681 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131981 | 58 |
| Changzhou Borl Biotechnology Co.,LTD | 13606124132;13656121842 | luyan0021@163.com | China | 219 | 58 |