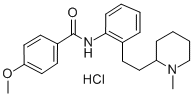
ENCAINIDE HYDROCHLORIDE
- CAS No.
- 66794-74-9
- Chemical Name:
- ENCAINIDE HYDROCHLORIDE
- Synonyms
- (+)-Isomer;CHEBI:59880;Encainide HCl;Encainide, (+)-Isomer;ENCAINIDE HYDROCHLORIDE;MJ-9067, (+/-)-4-Methoxy-N-[2-[2-(1-methyl-2-piperidinyl)ethyl]phenyl]benzamide hydrochloride
- CBNumber:
- CB91011220
- Molecular Formula:
- C22H28N2O2.ClH
- Molecular Weight:
- 388.936
- MDL Number:
- MFCD01667688
- MOL File:
- 66794-74-9.mol
- MSDS File:
- SDS
| Melting point | 131.5-132.5 °C |
|---|---|
| storage temp. | 2-8°C |
| solubility | H2O: >25mg/mL |
| form | powder |
| FDA UNII | 4CH7J36N9S |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H301-H315-H319-H335 |
| Precautionary statements | P301+P310+P330-P302+P352-P305+P351+P338 |
| Hazard Codes | T |
| Risk Statements | 25-36/37/38 |
| Safety Statements | 26-45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | CV5521500 |
| Toxicity | LD50 in mice, dogs (mg/kg): 86, 43 orally; 16, 17 i.v. (Mitchell, Winkle) |
ENCAINIDE HYDROCHLORIDE price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | E9156 | Encainide hydrochloride ≥98% (HPLC), powder | 66794-74-9 | 10mg | $218 | 2022-05-15 | Buy |
| Sigma-Aldrich | E9156 | Encainide hydrochloride ≥98% (HPLC), powder | 66794-74-9 | 50mg | $863 | 2022-05-15 | Buy |
| TRC | E555475 | Encainide hydrochloride | 66794-74-9 | 10mg | $265 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0007808 | ENCAINIDE HYDROCHLORIDE 95.00% | 66794-74-9 | 10MG | $713.71 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0007808 | ENCAINIDE HYDROCHLORIDE 95.00% | 66794-74-9 | 50MG | $1220.03 | 2021-12-16 | Buy |
ENCAINIDE HYDROCHLORIDE Chemical Properties,Uses,Production
Description
Encainide is an oral and injectable antiarrhythmic structurally related to flecainide. It is reportedly useful in the treatment of symptomatic and life-threatening ventricular arrhythmias. While it appears to have higher efficacy than quinine, the morbidity and mortality rates are comparable.
Originator
Bristol-Myers (USA)
Uses
Cardiac depressant (anti-arrhythmic.
Definition
ChEBI: The hydrochloride salt of encainide. A class Ic antiarrhythmic, it was used for the treatment of severe or life-threatening ventricular arrhythmias, but it was associated with increased death rates in patients who had asymptomatic heart rhythm abnormalitie after a recent heart attack and was withdrawn from the market.
Manufacturing Process
Encainide was prepared in three steps starting with methylantranylate.
A solution of 529.8 g (3.505 mole) methyl anthranilate and 294.4 g 50 weight
percent NaOH (3.68 mole) in 3.6 L CH2Cl2 and 1.8 L water was stirred in an
ice-bath as 627.8 g (3.680 mole) p-anisoyl chloride was added at such rate
that the temperature did not exceed 10°C (time required was 1.25 hr). The
mixture was allowed to warm to 23°C. Acetic acid (50 mL) was added to
adjust the pH to 5. The layers were separated and the organic layer was
washed with 10% aqueous NaHCO3 (1 times 0.8 L) and brine (1 times 0.8 L).
The residual white solid was recrystallized from 7.0 L boiling material. The
product was dried in vacuo at 70°C for 24 hr to yield 959.7 g (96.0%) white
crystalline solid, melting point 122.5°C-124.5°C.
2-(2-Pyridylacetyl)-p-anilsanilide. A dry, nitrogen purged flask was charged
with 1.875 ml 1.6 N (3.0 mole) n-butyl lithium in hexane. The solution was
stirred under nitrogen and chilled to -45° to -40°C, and 1.5 L THF (dried over
molecular sieve 4 A) was added slowly. Diisopropylamine (303.6 g; 3.0 mole)
was added at such a rate that the temperature did not exceed -30°C. Then
307.3 g (3.3 mole) 2-picoline was added with stirring, keeping the
temperature below -30°C. The cooling was interrupted, and the mixture was
slowly warmed to 10°C by which time the conversion to anion was complete
and all the 2-picolyl lithium had redissolved. The solution was recooled to -
45°C to -40°C (the orange solid reprecipitated), and a solution of 285.3 g (1.0
mole) methyl N-p-anisoylanthranilate in 1.9 L dry THF was added at a rate so
the temperature did not exceed -30°C. After the addition, the mixture was
slowly warmed to 25°C. The solution was adjusted to pH 6 with 500 mL acetic
acid; 5.0 H2O was added with stirring. Then the organic solvents were distilled
in vacuo and the residual yellow semi-solid product was extracted with CH2Cl2
(1 times 2.5 L). The extract was washed with H2O (1 times 1.0 L) and
stripped to dryness in vacuo. The residue was dissolved in 6.7 L boiling
isopropanol. The solution was chilled with stirring to 5°C, and the resulting
yellow solid was collected on a filter, rinsed with isopropanol and dried in
vacuo at 80°C for six hours. The filtrate was concentrated and chilled to yield
a second crop of product. Both crops of intensely yellow material exhibited
single spots in the TLC (7.5 cm silica gel with indicator, 9 CH2Cl2/1 methanol,
UV). The total yield was 306.7 g (88.5%) of material, m.p 145°-148.5°C.
2-(2-Pyridylacetyl)-p-anisanilide (25.0 g, 0.0722 mole) was dissolved withgentle warming in 500 ml THF. The bright yellow solution was chilled in an ice
bath, and 6.5 ml (0.078 mole) 12 N HCl was added. The yellow color
disappeared and a white precipitate formed immediately. The solid was
collected on a filter; rinsed with THF and air-dried to give 27.4 g white solid
2-(2-pyridylacetyl)-p-anisanilide hydrochloride (99.3%), m.p. 190.5°-191.5°C.
(dec.).
4-Methoxy-2'-[2-(1-methyl-2-piperidyl)-ethyl]benzanilide, Encainide. A
mixture of 53.5 (0.1397 mole) 2-(2-pyridylacetyl)-p-anisanilide hydrochloride,
1.0 g platinum catalyst (2.5-5% Pt/C or PtO2) and 1.0 L glacial acetic acid
was stirred vigorously under a slight positive pressure of H2 at 23°-25°C for
20 hr, by which time 0.43 mole (3.08 equivalents) of H2O had been absorbed.
The catalyst was removed by filtration through a celite bed. The filtrate was
returned to the flask, and 10.0 g 10% Pd/C was added under nitrogen. The
mixture was stirred vigorously under H2 as it was heated to 60°C. After an
additional 6.5 hr the total H2 uptake equaled 0.71 mole (5.08 equivalents,
101.6% of theory). The mixture was cooled to 25°C, and 22.7 g formalin (37
weight percent formaldehyde, 8.4 g, 0.28 mole) was injected into the reaction
mixture. The mixture was stirred vigorously under H2 at 23°-25°C for 20 hr;
during that time 0.1452 mole (1.04 equivalents) H2 was absorbed. The
catalyst was removed by filtration, and the filtrate concentrated in vacuo to a
thick oil. Twice the oil was mixed with 200 mL isopropanol and stripped in
vacuo at 90°C to a thick oil. The oil was dissolved in 200 mL boiling
isopropanol. The solution was stirred, seeded with, and chilled to 10°C for 1
hr. The solid was collected on a filter, rinsed with cold isopropanol (2 times 2.0
mL) to give 36.6 g (67.4%) product, m.p. 181.5°-184.5°C. Additional product
was obtained from isopropanol filtrate to give a total yield of 76.1% encainide.
brand name
Enkaid (Bristol Labs).
Therapeutic Function
Antiarrhythmic
Biochem/physiol Actions
Encainide hydrochloride is a sodium channel blocker and class Ic antiarrhythmic. Encainide is a non-chiral antiarrhythmic and benzanilide derivative.
ENCAINIDE HYDROCHLORIDE Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
ENCAINIDE HYDROCHLORIDE Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 | support@targetmol.com | United States | 19973 | 58 |
| AdooQ BioScience, LLC | +1 (866) 930-6790 | info@adooq.com | United States | 2784 | 58 |
| Sigma-Aldrich | 021-61415566 800-8193336 | orderCN@merckgroup.com | China | 51471 | 80 |
| Energy Chemical | 021-58432009 400-005-6266 | marketing@energy-chemical.com | China | 44941 | 58 |
| TargetMol Chemicals Inc. | 4008200310 | marketing@tsbiochem.com | China | 24131 | 58 |
| Supplier | Advantage |
|---|---|
| TargetMol Chemicals Inc. | 58 |
| AdooQ BioScience, LLC | 58 |
| Sigma-Aldrich | 80 |
| Energy Chemical | 58 |
| TargetMol Chemicals Inc. | 58 |