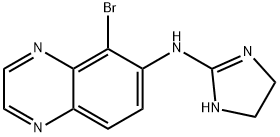
Brimonidine
- CAS No.
- 59803-98-4
- Chemical Name:
- Brimonidine
- Synonyms
- UK 14,304;UBC;UK 14;RPS27A;CS-1362;UK 14,34;UK-11957;lk14304-18;AGN-190342;BRIMONIDINE
- CBNumber:
- CB9111370
- Molecular Formula:
- C11H10BrN5
- Molecular Weight:
- 292.13
- MDL Number:
- MFCD00153878
- MOL File:
- 59803-98-4.mol
| Melting point | 207.5 °C |
|---|---|
| Boiling point | 432.6±55.0 °C(Predicted) |
| Density | 1.82±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: <0.8 mg/mL |
| form | powder to crystal |
| pka | 7.69±0.10(Predicted) |
| color | Light yellow to Amber to Dark green |
| Merck | 14,1375 |
| CAS DataBase Reference | 59803-98-4(CAS DataBase Reference) |
| FDA UNII | E6GNX3HHTE |
| ATC code | D11AX21,S01EA05,S01GA07 |
Brimonidine price More Price(65)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | U104 | UK 14,304 ≥98% (HPLC) | 59803-98-4 | 5mg | $90 | 2024-03-01 | Buy |
| Sigma-Aldrich | U104 | UK 14,304 ≥98% (HPLC) | 59803-98-4 | 25mg | $329 | 2024-03-01 | Buy |
| Sigma-Aldrich | WH0007314M1 | Monoclonal Anti-UBB antibody produced in mouse clone 1F5, purified immunoglobulin, buffered aqueous solution | 100μG | $524 | 2024-03-01 | Buy | |
| TCI Chemical | B4132 | Brimonidine >98.0%(HPLC)(T) | 59803-98-4 | 1g | $492 | 2024-03-01 | Buy |
| TCI Chemical | B4132 | Brimonidine >98.0%(HPLC)(T) | 59803-98-4 | 200mg | $144 | 2024-03-01 | Buy |
Brimonidine Chemical Properties,Uses,Production
Description
Launched in the US for open-angle glaucoma and ocular hypertension, brimonidine is a relatively selective and potent α2a,-adrenergic agonist with low affinity for the imidazoline l1 receptor. Topical application reduces intraocular pressure. This bypasses any central hypotensive effects at the l1 receptor (which can also give rise to a decrease in blood pressure and heart rate) if given systemically, since topical application results in low plasma levels concomitant with rapid systemic elimination. Brimonidine can be prepared in a four-step sequence from 4-nitrophenylenediamine.
Chemical Properties
Yellow Solid
Originator
Allergan (USA)
Uses
a2-Adrenoceptor agonist. Antiglaucoma
Uses
α2-Adrenoceptor agonist. Antiglaucoma.
Uses
coronary vasodilator calcium ion influx inhibitor
Definition
ChEBI: Brimonidine is a quinoxaline derivative, a secondary amine and a member of imidazoles. It has a role as an adrenergic agonist, an antihypertensive agent and an alpha-adrenergic agonist.
Manufacturing Process
6-Aminoquinoxaline (2.08 g, 14.4 mmol) was dissolved in 11.5 ml glacial
acetic acid. The solution was cooled in water while a solution of bromine (0.74
ml, 2.3 g, 14.4 mmol) in 1.5 ml glacial acetic acid was added slowly over 15
min. After stirring for an additional 30 min. the orange red solid formed was
filtered off and washed thoroughly with dry ether. The solid was dried in vacuo
overnight to yield 4.44 g crude product (a yield of 100%). The compound, 6-
amino-5-bromoquinoxaline hydrobromide, had no definite melting point. A phase change (from fine powder to red crystals) was noticed at about 220°C.
Decomposition was observed at about 245°C. It was used directly for the next
step.
The crude 6-amino-5-bromoquinoxaline from above was dissolved in water
and saturated sodium bisulfite solution was added until the resulting solution
tested negative with starch-iodide paper. The solution was then basified with 2
N sodium hydroxide and extracted thoroughly with ethyl acetate. The organic
extract was dried over magnesium sulfate and concentrated under reduced
pressure to give the free base. The crude product was recrystallized from
boiling benzene to give yellow crystals, m.p. 155°-156°C. Using various
analytical procedures, the yellow crystals were determined to be 6-amino-5-
bromoquinoxaline. The yield was 82%.
The crude hydrobromide product previously noted (4.27 g, 14.0 mmol) was
dissolved in 60 ml of water and thiophosgene (1.28 ml, 16.8 mmol) was
added in small portions with vigorous stirring. After 2 hours, the red color of
the solution was discharged. The solid formed was filtered off and washed
thoroughly with water. After drying in vacuo at 25°C 3.38 g (a yield of 90%)
of brick red crystals was obtained, m.p. 157°-158°C. A portion of this material
was further purified by column chromatography to give white crystals, m.p.
157°-158°C. Using various analytical procedures, these crystals were
determined to be 5-bromo-6-isothiocyanatoquinoxaline.
A solution of the isothiocyanate (3.25 g, 12.2 mmol) in 145 ml benzene was
added to a solution of ethylenediamine (5.43 g, 90.0 mmol) in 18 ml benzene
at 25°C over 2 hours. After stirring for a further 30 min., the supernatant was
poured off. The oil which remained was washed by swirling with dry ether
three times and used directly for the next step. A portion of this product was
further purified by column chromatography (SiO2, CHCl3) for characterization.
A white solid was decomposed at 175°C. This white solid was determined to
be 5-bromo-6-(N-2-(aminoethyl)thioureido)quinoxaline.
The crude product from above was dissolved in 100 ml dry methanol and the
brown solution was refluxed for 19 hours until hydrogen sulfide gas was no
longer evolved. The mixture was cooled to room temperature and
concentrated to about 50 ml. The yellow solid was filtered off and dried in
vacuo; weight 2.52 g (a yield of 70%), m.p. 242°-244°C. As the crude
product was insoluble in most common organic solvents, initial purification
was achieved by an acid-base extraction procedure. 23 g of the crude product
was dissolved in 100 ml 0.5 N hydrochloric acid. The turbid yellow solution
was filtered to give a clear orange yellow solution which was extracted twice
with ethyl acetate (2x10 ml). The aqueous phase was cooled to 0°C and
basified with 6 N sodium hydroxide, keeping the temperature of the solution
below 15°C at all times. The yellow solid which precipitated was filtered off
and washed thoroughly with water until the washings were neutral to pH
paper. The solid was dried overnight in vacuo to give 1.97 g yellow solid, m.p.
249°-250°C. The recovery was about 88%.
Further purification was achieved by recrystallization as described below. The
partially purified product from above was dissolved in N,N-dimethylformamide
(about 17 ml/g) at 100°C with vigorous stirring. The solution was filtered hot
and set aside to cool overnight. The bright yellow crystals were collected by
filtration, m.p. 252°-253°C. Recovery was from 65-77%. Using various analytical procedures the bright yellow solid was determined to be 5-bromo-6-
(2-imidazolin-2-ylamino)quinoxaline.
brand name
Alphagan (Allergan).
Therapeutic Function
Antiglaucoma
Hazard
A poison by ingestion.
Biological Activity
Full α 2 adrenergic agonist. Centrally active following systemic administration in vivo . Also available as part of the α 2 -Adrenoceptor Tocriset™ and Mixed Adrenergic Tocriset™ .
Biochem/physiol Actions
UK 14,304 is an α2-adrenoceptor agonist. UK 14,304 inhibits hormone-sensitive lipase (HSL) activity and suppresses lipogenesis in adipose tissue.
Veterinary Drugs and Treatments
Brimonidine is an alpha-adrenergic receptor agonist. It has a peak ocular hypotensive effect occurring at two hours post-dosing. Fluorophotometric studies in animals and humans suggest that brimonidine tartrate has a dual mechanism of action by reducing aqueous humor production and by and increasing uveoscleral outflow. After ocular administration of either a 0.1% or 0.2% solution, plasma concentrations peaked within 0.5 to 2.5 hours and declined with a systemic half-life of approximately 2 hours. In humans, systemic metabolism of brimonidine is extensive. It is metabolized primarily by the liver. Urinary excretion is the major route of elimination of the drug and its metabolites. Approximately 87% of an orally-administered radioactive dose was eliminated within 120 hours, with 74% found in the urine.
storage
Store at RT
Brimonidine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12457 | 58 |
| Zhengzhou Anbu Chem Co.,Ltd | +86-0371-88006763; +8615988602810 | sales@anbuchem.com | China | 3000 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shanghai Yingrui Biopharma Co.,Ltd | 21-33585366 | export01@shyrchem.com | CHINA | 1320 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Guangzhou Yuheng Pharmaceutical Technology Co., Ltd | +8613580539051 | joe@yuhengpharm.com | CHINA | 21149 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
View Lastest Price from Brimonidine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-17 | Brimonidine
59803-98-4
|
US $1.10 / g | 1g | 99.0% min | 100 tons min | Shaanxi Dideu Medichem Co. Ltd | |
 |
2023-09-20 | 5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)quinoxalin-6-amine
59803-98-4
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-07-15 | Brimonidine
59803-98-4
|
US $0.00 / KG | 0.1KG | 98% | 1000KGS | Zhengzhou Anbu Chem Co.,Ltd |
-

- Brimonidine
59803-98-4
- US $1.10 / g
- 99.0% min
- Shaanxi Dideu Medichem Co. Ltd
-

- 5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)quinoxalin-6-amine
59803-98-4
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Brimonidine
59803-98-4
- US $0.00 / KG
- 98%
- Zhengzhou Anbu Chem Co.,Ltd