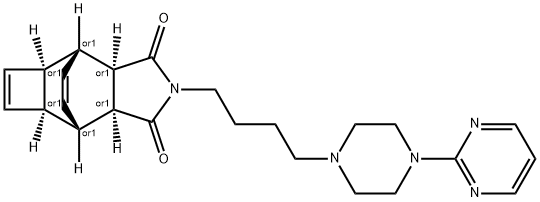
zalospirone
- CAS No.
- 114298-18-9
- Chemical Name:
- zalospirone
- Synonyms
- zalospirone;2-<4-<4-(2-pyrimidinyl)-1-piperazinyl>butyl>hexahydro-4,7-etheno-1H-cyclobut
isoindole-1,3(2H)-dione;4,7-Etheno-1H-cyclobut[f]isoindole-1,3(2H)-dione, 3a,4,4a,6a,7,7a-hexahydro-2-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-, (3aR,4R,4aR,6aS,7S,7aS)-rel-
- CBNumber:
- CB91370819
- Molecular Formula:
- C24H29N5O2
- Molecular Weight:
- 419.52
- MDL Number:
- MOL File:
- 114298-18-9.mol
| FDA UNII | 0449O95Z1J |
|---|
zalospirone Chemical Properties,Uses,Production
Originator
Zalospirone hydrochloride,American Home Products (AHP)
Uses
Anti-anxiety agent.
Manufacturing Process
To a stirred solution of 0.035 mole of 1,3-dioxo-4,7-etheno-δ 5 -1,3,3a,7a- tetrahydrocyclobut[f]isoindole in 70 ml of dimethylformamide is added 0.9 g of sodium hydride. The suspension is stirred at 60°C for 3 hours and is poured into a stirred solution of 1,4-dibromobutane (0.04 mol) in 50 ml of dimethylformamide. The reaction mixture is stirred at room temperature for 24 hours, dimethylformamide is evaporated under reduced pressure and the residue is extracted with methylene chloride (3x200 ml). The methylene chloride extracts are collected, washed with water, dried over anhydrous Na 2 SO 4 and evaporated under reduced pressure. The residue is solidified to a waxy like material. 0.007 mole of this material is dissolved in 50 ml of dimethylformamide, and to this solution is added 6 ml of triethylamine and 0.007 mole of 1-(2-pyrimidinyl)piperazine dihydrochloride. The reaction mixture is stirred at room temperature for 48 hours. Dimethylformamide is removed under reduced pressure and the remaining solid is extracted with 2x100 ml of methylene chloride. The methylene chloride extracts are dried over anhydrous Na 2 SO 4 , evaporated and the residue is separated by HPLC using 30% methanolethyl acetate as eluent. Evaporation of the solvent from the desired fractions (R f = 0.5) affords the 3a,4,4a,6a,7,7a-hexahydro-2-[4- [4-(2-pyrimidinyl)-1-piperazinyl]butyl]-4,7-etheno-1H-cyclobut[f]isoindole- 1,3(2H)-dione dihydrochloride, which is converted to the dihydrochloride salt by dissolving the free base in ethanol and adding ether saturated with hydrogen chloride; MP: 252°-254°C.
Therapeutic Function
Anxiolytic
zalospirone Preparation Products And Raw materials
Raw materials
Preparation Products
zalospirone Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Changzhou Bojia Biomedical Technology Co., Ltd. | 2122619822 | czbjpharma@126.com | China | 18488 | 58 |
| Supplier | Advantage |
|---|---|
| Changzhou Bojia Biomedical Technology Co., Ltd. | 58 |