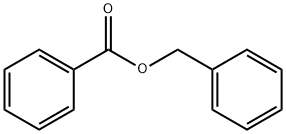
Benzyl benzoate
- CAS No.
- 120-51-4
- Chemical Name:
- Benzyl benzoate
- Synonyms
- benzyl benzonate;Benzyl ester;ASCABIOL;Benzylbenzoat;Benzyl Benoate;BENZOATO DE BENCILO;Nat.Benzyl benzoate;BENZOIC ACID BENZYL ESTER;BENZOIC ACID PHENYLMETHYL ESTER;Benzoic acid benzyl ester≥ 99% (GC)
- CBNumber:
- CB9152283
- Molecular Formula:
- C14H12O2
- Molecular Weight:
- 212.24
- MDL Number:
- MFCD00003075
- MOL File:
- 120-51-4.mol
- MSDS File:
- SDS
| Melting point | 17-20 °C (lit.) |
|---|---|
| Boiling point | 323-324 °C (lit.) |
| Density | 1.118 g/mL at 20 °C (lit.) |
| vapor pressure | 1 mm Hg ( 125 °C) |
| refractive index |
n |
| FEMA | 2138 | BENZYL BENZOATE |
| Flash point | 298 °F |
| storage temp. | 2-8°C |
| solubility | Miscible with ethanol, alcohol, chloroform, ether, oils. |
| form | Liquid |
| color | Clear colorless |
| Odor | at 100.00 %. faint sweet balsam oily herbal |
| Odor Type | balsamic |
| Water Solubility | practically insoluble |
| Merck | 14,1127 |
| JECFA Number | 24 |
| BRN | 2049280 |
| Dielectric constant | 4.8(20℃) |
| Stability | Stable. Substances to be avoided include strong oxidizing agents. Combustible. |
| InChIKey | SESFRYSPDFLNCH-UHFFFAOYSA-N |
| LogP | 4 at 20℃ |
| CAS DataBase Reference | 120-51-4(CAS DataBase Reference) |
| Substances Added to Food (formerly EAFUS) | BENZYL BENZOATE |
| FDA 21 CFR | 172.515; 175.105; 310.545 |
| EWG's Food Scores | 3-6 |
| FDA UNII | N863NB338G |
| NIST Chemistry Reference | Benzyl Benzoate(120-51-4) |
| ATC code | P03AX01 |
| Pesticides Freedom of Information Act (FOIA) | Benzyl benzoate |
| EPA Substance Registry System | Benzyl benzoate (120-51-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H411 | |||||||||
| Precautionary statements | P264-P270-P273-P301+P312-P391-P501 | |||||||||
| Hazard Codes | Xn,N | |||||||||
| Risk Statements | 22-51/53 | |||||||||
| Safety Statements | 25-61-46 | |||||||||
| RIDADR | UN 3082 9 / PGIII | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | DG4200000 | |||||||||
| Autoignition Temperature | 896 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| HS Code | 29163100 | |||||||||
| Toxicity | LD50 in rats, mice, rabbits, guinea pigs (g/kg): 1.7, 1.4, 1.8, 1.0 orally (Draize) | |||||||||
| NFPA 704 |
|
Benzyl benzoate price More Price(62)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W213810 | Benzyl Benzoate natural, ≥99%, FCC, FG | 120-51-4 | 100g | $127 | 2024-03-01 | Buy |
| Sigma-Aldrich | W213810 | Benzyl Benzoate natural, ≥99%, FCC, FG | 120-51-4 | 1kg | $662 | 2024-03-01 | Buy |
| Sigma-Aldrich | W213802 | Benzyl Benzoate ≥99%, FCC, FG | 120-51-4 | 1kg | $76.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | W213802 | Benzyl Benzoate ≥99%, FCC, FG | 120-51-4 | 5kg | $160 | 2024-03-01 | Buy |
| Sigma-Aldrich | W213802 | Benzyl Benzoate ≥99%, FCC, FG | 120-51-4 | 10Kg | $214 | 2024-03-01 | Buy |
Benzyl benzoate Chemical Properties,Uses,Production
Description
Benzyl benzoate (BnBzO) is a mediation and insect repellent. It is one of the older preparation used to treat scabies which is a skin infections caused by the mite scarcoptes scabiei since it is lethal to the mite. It is capable of killing the mite in 5 minutes. It can also be used for the treatment of lice infestation of the head and the body. Its mechanism of action is through exerting toxic effect on the nervous system of the insects, further causing its death. It is also toxic to mite ova through an unknown mechanism. It can also be used as a repellent for chiggers, ticks, and mosquitoes as well as a dye carrier, solvent of cellulose derivatives, plasticizer, and a fixative.
Chemical Properties
Benzyl benzoate is a clear, colorless, oily liquid with a light, balsamic odor reminiscent of almond and a sharp, pungent taste. It produces a sharp, burning sensation on the tongue. At temperatures below 178℃ it exists as clear, colorless crystals.
Uses
Benzyl Benzoate has seen use as an insecticide, as well as a solvent for various chemical reactions.
Benzyl benzoate is a benzyl compound that can be synthesized by reacting benzyl chloride with sodium benzoate in the presence of tetrabutylaramonium iodide. It has been used to:
Prepare benzyl alcohol/benzyl benzoate (BABB) solution employed for enhancing the tissue transparency during the whole-mount immunostaining BABB method.
Study its ability to induce an olfactory response in the third instar larvae of Drosophila melanogaster.
Prepare Spalteholz fluid in combination with methyl salicylate, employed during LSFM (light sheet fluorescence microscopy) imaging.
Preparation
Benzyl benzoate is a constituent of Peru balsam and occurs naturally in certain plant species. Commercially, benzyl benzoate is produced synthetically by the dry esterification of sodium benzoate and benzoyl chloride in the presence of triethylamine or by the reaction of sodium benzylate with benzaldehyde.
toxicity
In man, benzyl benzoate was a skin sensitizer and caused gastro-intestinal effects in an infant. It was of moderate to low acute oral toxicity in a range of laboratory animal species and of low acute dermal toxicity in rabbits. Central nervous system effects were induced in several species given single doses orally or dermally. Repeated skin applications caused degenerative changes in various organs (including the liver, spleen and testes) of rabbits. No effects were seen in reproductive studies involving repeated oral administration to rats during pregnancy and lactation. Benzyl benzoate gave no evidence of mutagenicity in an Ames bacterial assay. It was a mild skin irritant in rabbits.
References
https://www.drugbank.ca/drugs/DB00676
https://en.wikipedia.org/wiki/Benzyl_benzoate
Description
Benzyl benzoate is the ester of benzyl alcohol and benzoic acid, with the formula C6H5CH2O2CC6H5. This easily prepared compound with a mild balsamic odor has a variety of uses.Benzyl benzoate (BB) is one of the oldest drugs used for the treatment of scabies and is recommended as the “first-line intervention” for the cost-effective treatment of the disease.
Chemical Properties
Benzyl Benzoate is the main component of Peru balsam oil. It occurs in fairly large amounts in a number of blossom concretes and absolutes (e.g., tuberose and hyacinth). It forms either a viscous liquid or solid flakes (mp 21–22°C) and has a weak, sweet, balsamic odor.

Benzyl Benzoate is prepared either by transesterification of technical methyl benzoate with benzyl alcohol or from benzyl chloride and sodium benzoate. A third process starts with benzaldehyde, which is converted in high yield into benzyl benzoate in the presence of sodium or aluminum benzylate (Tishchenko reaction). Benzyl benzoate is used in perfumery as a fixative and as a modifier in heavy blossom fragrances.
Occurrence
Contained in Peru balsam and in the concrete and absolute of tuberose flowers, hyacinth, Narcissus jonquilla L., and Dianthus caryophillus L.; also in the oil of ylang-ylang and in Tolu balsam. Reported found in American cranberry, cinnamon bark, cassia leaf, corn oil and hog plum (Spondias mombins L.).
Uses
As solvent of cellulose acetate, nitrocellulose and artificial musk; substitute for camphor in celluloid and plastic pyroxylin Compounds; perfume fixative; in confectionery and chewing gum flavors.
Uses
Benzyl benzoate, as a topical solution, may be used as an antiparasitic insecticide to kill the mites responsible for the skin condition scabies , for example as a combination drug of benzyl benzoate/disulfiram.
It has other uses :
a fixative in fragrances to improve the stability and other characteristics of the main ingredients
a food additive in artificial flavors
a plasticizer in cellulose and other polymers
a solvent for various chemical reactions
a treatment for sweet itch in horses
a treatment for scaly leg mites in chickens.
Uses
benzyl benzoate is an anti-microbial. It can also act as a solvent, helping dissolve other substances in the product, and as a perfuming ingredient. It is the ester of benzyl alcohol and benzoic acid.
Definition
ChEBI: Benzyl benzoate is a benzoate ester obtained by the formal condensation of benzoic acid with benzyl alcohol. It has been isolated from the plant species of the genus Polyalthia. It has a role as a scabicide, an acaricide and a plant metabolite. It is a benzyl ester and a benzoate ester. It is functionally related to a benzoic acid.
Definition
Benzyl benzoate is produced from benzyl alcohol and sodium benzoate in the presence of triethylamine or by transesterification of methyl benzoate with benzyl alcohol in the presence of an alkali benzyl oxide. In another manufacturing process benzaldehyde is condensed to form benzyl benzoate in the presence of sodium (Claisen-Tishchenko condensation). The presence of a small amount of an aliphatic ether improves this reaction. Benzyl benzoate is a byproduct in the manufacture of benzoic acid by the oxidation of toluene; it is present in the benzoic acid distillation residue.
Indications
Benzyl benzoate: 20% to 25%. This agent is relatively nontoxic and is widely
used in developing countries to treat scabies and pediculosis capitis and pubis.
Only a veterinary preparation is available in the United States. Benzyl benzoate is
synthetically derived from the esterification of benzoic acid with benzyl alcohol. Its
mechanism of action is unknown. It is toxic to Sarcoptes scabei and may be toxic
to Pediculosis capitis and Phthirus pubis. No resistance has been demonstrated
to date.
Benzyl benzoate can be used in a 5% emulsion to repel many arthropods and can
be used as a lotion to treat sarcoptic mange and canine pediculosis.
Production Methods
BENZYL BENZOATE is produced by the Cannizzaro reaction from benzaldehyde, by esterifying benzyl alcohol with benzoic acid, or by treating sodium benzoate with benzyl chloride. It is purified by distillation and crystallization. Benzyl benzoate is used as a fixative and solvent for musk in perfumes and flavors, as a plasticizer, miticide, and in some external medications. The compound has been found effective in the treatment of scabies and pediculosis capitis (head lice, Pediculus humanus var. capitis).
Preparation
By the dry esterification of sodium benzoate and benzoyl chloride in the presence of triethylamine or by reaction of sodium benzylate on benzaldehyde.
brand name
Pharmaceutic necessity for Dimercaprol [Injection]. Benylate (Sterling Winthrop).
Taste threshold values
Taste characteristics at 30 ppm: balsamic, fruity with powdery and berry nuances.
Synthesis Reference(s)
Journal of Heterocyclic Chemistry, 24, p. 187, 1987 DOI: 10.1002/jhet.5570240135
Tetrahedron Letters, 27, p. 2383, 1986 DOI: 10.1016/S0040-4039(00)84535-9
General Description
Benzyl benzoate is an aromatic ester that is used as a food flavoring agent. It has been identified as one of the main volatile aroma component of cranberry, mango and Egyptian Jasminum sambac flowers.
Hazard
Irritant to eyes, skin.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Benzyl benzoate is used as a solubilizing agent and nonaqueous
solvent in intramuscular injections at concentrations of
0.01–46.0% v/v, and as a solvent and plasticizer for cellulose
and nitrocellulose. It is also used in the preparation of spray-dried
powders using nanocapsules.
However, the most widespread pharmaceutical use of benzyl
benzoate is as a topical therapeutic agent in the treatment of
scabies. Benzyl benzoate is also used therapeutically as a
parasiticide in veterinary medicine.
Other applications of benzyl benzoate include its use as a
pediculicide, and as a solvent and fixative for flavors and perfumes
in cosmetics and food products.
Biochem/physiol Actions
Benzyl benzoate is the condensation product of benzoic acid and benzyl alcohol. It is utilized for treating human scabies as well as kills house dust mite.
Contact allergens
Benzyl benzoate is the ester of benzyl alcohol and benzoic acid. It is contained in Myroxylon pereirae and Tolu balsam. It is used in acaricide preparations against Sarcoptes scabiei or as a pediculicide. Direct contact may cause skin irritation, but rarely allergic contact dermatitis. As a fragrance allergen, benzyl benzoate has to be mentioned by name in EU cosmetics.
Clinical Use
Benzyl benzoate is a naturally occurring ester obtained fromPeru balsam and other resins. It is also prepared syntheticallyfrom benzyl alcohol and benzoyl chloride. The ester isa clear colorless liquid with a faint aromatic odor. It is insolublein water but soluble in organic solvents.
Benzyl benzoate is an effective scabicide when appliedtopically. Immediate relief from itching probably resultsfrom a local anesthetic effect; however, a complete cureis frequently achieved with a single application of a 25%emulsion of benzyl benzoate in oleic acid, stabilized withtriethanolamine. This preparation has the additionaladvantage of being essentially odorless, nonstaining, andnonirritating to the skin. It is applied topically as a lotionover the entire dampened body, except the face.
Safety
Benzyl benzoate is metabolized by rapid hydrolysis to benzoic acid
and benzyl alcohol. Benzyl alcohol is then further metabolized to
hippuric acid, which is excreted in the urine.
Benzyl benzoate is widely used as a 25% v/v topical application
in the treatment of scabies and as an excipient in intramuscular
injections and oral products. Adverse reactions to benzyl benzoate
include skin irritation and hypersensitivity reactions. Oral ingestion
may cause harmful stimulation of the CNS and convulsions. Benzyl
benzoate should be avoided by perople with perfume allergy.
LD50 (cat, oral): 2.24 g/kg
LD50 (dog, oral): 22.44 g/kg
LD50 (guinea pig, oral): 1.0 g/kg
LD50 (mouse, oral): 1.4 g/kg
LD50 (rabbit, oral): 1.68 g/kg
LD50 (rabbit, skin): 4.0 g/kg
LD50 (rat, oral): 0.5 g/kg
LD50 (rat, skin): 4.0 g/kg
Synthesis
This colorless liquid is formally the condensation product of benzoic acid and benzyl alcohol. It can also be generated from benzaldehyde by the Tishchenko reaction.
Environmental Fate
Benzyl benzoate acts as a local irritant. At high levels of exposure, free benzoic acid may sequester significant amounts of acetyl coenzyme A (CoA), which could disrupt cholinergic signaling. Recent findings suggest that benzyl benzoate may have estrogenic properties.
Metabolism
Benzyl benzoate, a relatively non-toxic liquid widely used for the treatment of scabies, is converted into benzoic acid in vivo (Williams, 1959).
storage
Benzyl benzoate is stable when stored in tight, well-filled, lightresistant containers. Exposure to excessive heat (above 408℃) should be avoided.
Toxicity evaluation
Acute oral LD50 for rats: 1,700 mg/kg
Incompatibilities
Benzyl benzoate is incompatible with alkalis and oxidizing agents.
Regulatory Status
Included in the FDA Inactive Ingredients Database (IM injections and oral capsules). Included, as an active ingredient, in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Benzyl benzoate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhan Biet Co., Ltd. | +8617320528784 | min@biet.com.cn | China | 41 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei Aumei New Material Co., Ltd. | +86-027-87366298 +8615927270571 | tianyi@skbiology.cn | China | 294 | 58 |
| Shijiazhuang Tongyang Import and Export Co., LTD | 18031361688; | admin@sjztongyang.com | China | 987 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418684 +8618949823763 | sales@tnjchem.com | China | 25363 | 58 |
| Jinan Qinmu Fine Chemical Co.,Ltd. | +8618660799346 | sales@qinmuchem.com | China | 1009 | 58 |
| Hu Bei Jiutian Bio-medical Technology CO.,Ltd | 027-88013699 17354350817 | Ryan@jiutian-bio.com | China | 7433 | 58 |
| Wuhan Boyuan Import & Export Co., LTD | +8615175982296 | Mike@whby-chem.com | China | 974 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Dorne Chemical Technology co. LTD | +86-13583358881 +86-18560316533 | Ethan@dornechem.com | China | 293 | 58 |
Related articles
- The preparation method of benzyl benzoate
- Benzyl benzoate (BB) is one of the oldest drugs used for the treatment of scabies and is recommended as the “first-line interv....
- Aug 12,2022
- Side effects of Benzyl benzoate
- Benzyl benzoate occurs naturally in Peruvian and Tulu balsams as well as other essential oils. It was used in Vietnam War as a....
- Nov 23,2021
View Lastest Price from Benzyl benzoate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-19 | Benzyl benzoate
120-51-4
|
US $3.00-1.00 / kg | 1kg | 99.9% | 10 tons | Shanghai Aosiris new Material Technology Co., LTD | |
 |
2024-04-19 | Benzyl benzoate
120-51-4
|
US $50.00 / kg | 1kg | 99.10% | 5000kg | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-19 | Benzyl benzoate
120-51-4
|
US $1.00 / g | 1g | 99% | 1000kg | Dorne Chemical Technology co. LTD |
-

- Benzyl benzoate
120-51-4
- US $3.00-1.00 / kg
- 99.9%
- Shanghai Aosiris new Material Technology Co., LTD
-

- Benzyl benzoate
120-51-4
- US $50.00 / kg
- 99.10%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- Benzyl benzoate
120-51-4
- US $1.00 / g
- 99%
- Dorne Chemical Technology co. LTD