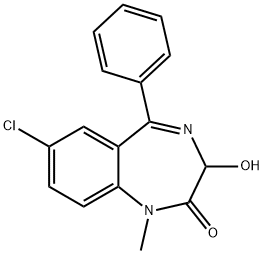
TEMAZEPAM
- CAS No.
- 846-50-4
- Chemical Name:
- TEMAZEPAM
- Synonyms
- er115;k3917;wy3917;wy2917;Planum;ER 115;K-3917;Wy-3917;WY 2917;Cerepax
- CBNumber:
- CB9185627
- Molecular Formula:
- C16H13ClN2O2
- Molecular Weight:
- 300.74
- MDL Number:
- MFCD00057904
- MOL File:
- 846-50-4.mol
| Melting point | 119-121°C |
|---|---|
| Boiling point | 211°C (rough estimate) |
| Density | 1.2682 (rough estimate) |
| refractive index | 1.5200 (estimate) |
| Flash point | 11 °C |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, freely soluble in methylene chloride, sparingly soluble in ethanol (96 per cent). |
| pka | pKa 1.6 (Uncertain) |
| BCS Class | 2 |
| CAS DataBase Reference | 846-50-4(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | CHB1QD2QSS |
| IARC | 3 (Vol. 66) 1996 |
| Proposition 65 List | Temazepam |
| ATC code | N05CD07 |
| EPA Substance Registry System | Temazepam (846-50-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P330-P501 | |||||||||
| Hazard Codes | Xn,T,F | |||||||||
| Risk Statements | 22-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 16-36/37-45-7 | |||||||||
| RIDADR | UN 1230 3/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | DF1225000 | |||||||||
| HS Code | 2933910000 | |||||||||
| Toxicity | LD50 oral in rat: 2gm/kg | |||||||||
| NFPA 704 |
|
TEMAZEPAM Chemical Properties,Uses,Production
Chemical Properties
Crystalline Solid
Originator
Levanxol,Carlo Erba,Italy,1970
Uses
Pharmacologically active labelled metabolite of Diazepam. Controlled substance (depressant). Sedative, hypnotic
Uses
Pharmacologically active metabolite of Diazepam. Controlled substance (depressant). Sedative, hypnotic
Uses
Temazepam, is pharmacologically active metabolite of Diazepam (D416855). It is Sedative, and hypnotic. Controlled substance (depressant).
Definition
ChEBI: Temazepam is a benzodiazepine.
Manufacturing Process
According to British Patent 1,022,645 3.4 g of 3-acetoxy-7-chloro-1-methyl-5- phenyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one suspended in 80 ml alcohol was treated with 6 ml of 4 N NaOH. After complete solution had taken place, a solid precipitated; this solid was redissolved by the addition of 80 ml of water. The solution was acidified with acetic acid to give white crystals which were recrystallized from alcohol to yield 7-chloro-3-hydroxy-5-phenyl-1- methyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one, MP 119° to 121°C.
brand name
Restoril (Tyco); Temaz (Quantum Pharmics).
Therapeutic Function
Tranquilizer
World Health Organization (WHO)
Temazepam is a widely used benzodiazepine derivative. As with other drugs in this class, cases of misuse and drug dependence are known.
General Description
Temazepam, 7-chloro-1,3-dihydro-3-hydroxy-1-methyl-5-phenyl -2H-1,4-benzodiazepine-2-one(Restoril), also occurs as a minor metabolite of diazepam.It can be visualized as N-methyl oxazepam, and indeed, asmall amount of N-demethylation occurs slowly. However,metabolism proceeds mainly through glucuronidationof the 3-hydroxyl group, thus, it is intermediate acting andmarketed as a hypnotic said to have little or no residualeffect.
Clinical Use
Benzodiazepine:
Insomnia (short-term use)
Pre-med anxiolytic prior to minor procedures
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: metabolism possibly increased by
rifampicin.
Antipsychotics: increased sedative effects; risk of
serious adverse effects in combination with clozapine.
Antivirals: concentration possibly increased by
ritonavir.
Disulfiram: metabolism of temazepam inhibited
(increased toxicity).
Sodium oxybate: enhanced effects of sodium oxybate
- avoid.
Metabolism
Temazepam is metabolised mainly in the liver. It is excreted mainly in the urine in the form of its inactive glucuronide conjugate together with small amounts of the demethylated derivative, oxazepam, also in conjugated form.
TEMAZEPAM Preparation Products And Raw materials
846-50-4(TEMAZEPAM)Related Search:
1of4