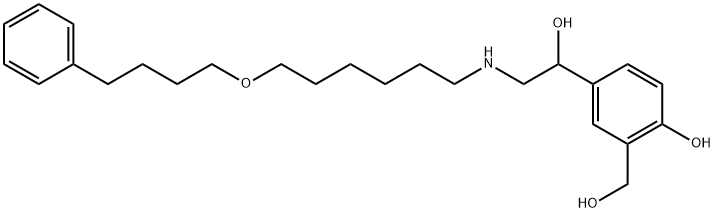
Salmeterol
- CAS No.
- 89365-50-4
- Chemical Name:
- Salmeterol
- Synonyms
- CS-380;SN408D;gr33343x;GR 33343;SAMETEROL;Astmerole;SALMETEROL;BNKY006-SX05;Salmeterol base;Salmeterol, >=99%
- CBNumber:
- CB9186866
- Molecular Formula:
- C25H37NO4
- Molecular Weight:
- 415.57
- MDL Number:
- MFCD00867037
- MOL File:
- 89365-50-4.mol
| Melting point | 75.5-76.5° |
|---|---|
| Boiling point | 603.0±55.0 °C(Predicted) |
| Density | 1.112±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| pka | 9.99±0.31(Predicted) |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 89365-50-4(CAS DataBase Reference) |
| FDA UNII | 2I4BC502BT |
| ATC code | R03AC12 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| RTECS | CZ6457000 |
Salmeterol price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | S090105 | Salmeterol | 89365-50-4 | 10mg | $130 | 2021-12-16 | Buy |
| Usbiological | S0058-65C | Salmeterol | 89365-50-4 | 500ul | $280 | 2021-12-16 | Buy |
| ChemScene | CS-2815 | Salmeterol 99.88% | 89365-50-4 | 50mg | $350 | 2021-12-16 | Buy |
| ChemScene | CS-2815 | Salmeterol 99.88% | 89365-50-4 | 10mg | $100 | 2021-12-16 | Buy |
| ChemScene | CS-2815 | Salmeterol 99.88% | 89365-50-4 | 100mg | $600 | 2021-12-16 | Buy |
Salmeterol Chemical Properties,Uses,Production
Asthma curative
Salmeterol is an asthma remedy. It is developed from the molecular structure of salbutamol with only an additional tail part. This part is closely associated with the specific structure of the beta 2 receptor, the external receptor site. This allows the other parts of the molecule to act freely on the beta 2 receptor. And for this reason, this product can continue to stay in the position of action. Salmeterol can be used as a bronchodilator for long-acting beta 2 receptor agonists in daily control of asthma symptoms. It can be used for long-term treatment of asthma (including nocturnal asthma and exercise-induced asthma), reversible airway obstruction in chronic bronchitis and emphysema, and is not suitable for acute attack of asthma.
Antiasthmatic medicine
Antiasthmatic drugs are the drugs that can act on the different links of the asthma attack in order to relieve or prevent the attack of asthma.
Salmeterol is a new long-acting beta 2 agonist antiasthmatic drug, derived from salbutamol, and closely linked to beta 2 receptor, resulting in more durable activation. Its chemical name is hydroxy naphthalate, which is white or white like powder with bitter taste. It was first developed by the British GlaxoSmithKline Co and was first listed in the UK in 1990.
Its commodity name is Serevent, Salmeterol Xinafoate Aerosol,Sertindole and Salmeterol. It can inhibit the release of hypersensitivity mediators from the mast cells in the lung. It can inhibit the early and late phase reaction induced by inhalation antigen and reduce the hyperresponsiveness of the trachea. Clinical trials show that is salmeterol stronger than salbutamol. It has a longer duration effect to protect asthma caused by histamine induced bronchoconstriction and movement. It features long duration, small extrapulmonary effect and good tolerance. It is an ideal drug for the treatment of nocturnal attack and asthma. The pharmacokinetic study has indicated that this dose of this product twice a day, 50μg each time. 5~15 min after 1 inhalation of 50 and 400μg, the highest plasma concentration will be 0.1 to 0.2μg /L and 1 ~ μg/L respectively. This product is strongly metabolizing by hydrolysis, and most of the dosage is eliminated in 72h. The doses of 23% and 57% in 168h can be found in urine and feces respectively. In addition, salmeterol has a controlled effect on histamine, leukotrienes and prostaglandins. It is worth notice that this product can not be used with non selective beta blockers for the treatment of asthma, and it should be prudent for patients with asthma with heart disease. Salmeterol 's common adverse reactions include hypokalemia, abnormal bronchospasm, tremor, headache, and palpitations. Its common beta agonists include: ephedrine, isoproterenol, salbutamol, terbutaline, clenbuterol, clorprenaline, tulobuterol, clenbuterol, procaterol, salmeterol, bambuterol, methoxyphenamine, rimiterol, bitolterol, sonarin, butero, fenoterol etc..
Clinical evaluation
It has been proved that this product dose is 50μg, inhaled 2 twice a day, and is effective for mild to moderate asthma. In patients with moderate asthma, it will be more efficient to take a dose of 100μg by inhalation twice a day. The effect is longer and up to 12 h.
It has been proved that the dose of 5050μg by inhaling twice a day will be more effectivethan the dose of 200μg salbutamol by inhaling 4 times a day in improving lung function and lightening the day and night respiratory syndrome. Salmeterol aerosol should used in adult bronchial asthma, 50μg each time, twice a day (morning, before bedtime), and the longest medication time is 71 weeks. The results shows an obvious tendency to improve compared with the pre medication. The improvement in the fourth week after administration is 15%. The moderate improvement is 50%, and the mild improvement is 72.5%. The improvement rate of long-term medication is 15%, 68.2% and 88.6%, respectively. 18 patients with bronchial asthma have been given aerosol 25μg and inhalation powder 25μg each time. The results have indicated that FEV10, PEFR, MMEF, V50 and V25 begin to be improved after 25 min, and their effects have maintained for 8h. The results of lung function examination after inhalation of 4h are the same.
Both the total improvement and the moderate improvement are both 81.8%, and the effectis completely equal. For the patients with asthma at night, inhalation of 50μg and 100μg every day for twice can obviously improve the expiratory flow rate throughout the night and increase the percentage of sleep in patients with nocturnal asthma.
These indexes are superior to placebo. Most studies have shown that this product improves the quality of sleep in the subjects.
Description
Salmeterol is a long-acting β2- agonist for patients with chronic asthma, useful also in controlling persistent nocturnal asthma.
Uses
Bronchodilator.
Uses
Salmeterol is a β2-Adrenergic agonist used for relief and control of asthma symptoms.
Definition
ChEBI: A phenol having a hydroxymethyl group at C-2 and a 1-hydroxy-2-{[6-(4-phenylbutoxy)hexyl]amino}ethyl group at C-4; derivative of phenylethanolamine.
General Description
Salmeterol has an N-phenylbutoxyhexyl substituent incombination with a β-OH group and a salicyl phenyl ring foroptimal direct-acting β2-receptor selectivity and potency. Ithas a potency similar to that of ISO. This drug associateswith the β2-receptor slowly resulting in slow onset of actionand dissociates from the receptor at an even slower rate. It is resistant to both MAO and COMT and highly lipophilic(log P=3.88). It is thus very long acting (12 hours), aneffect also attributed to the highly lipophilic phenylalkylsubstituent on the nitrogen atom, which is believed to interactwith a site outside but adjacent to the active site.
Hazard
A poison by inhalation.
Biological Activity
Potent and selective β 2 -adrenoceptor agonist (EC 50 = 5.3 nM); bronchodilator. Unlike other β 2 agonists, binds to exo-site domain of β 2 receptors, producing a slow onset of action and prolonged activation. Also available as part of the β -Adrenoceptor Agonist Tocriset™ .
Mechanism of action
A β2-agonist with a slow onset and extended duration of action is salmeterol. Salmeterol has the same phenyl ring substitution R3 as albuterol but also an unusually long and lipophilic group R1 on the nitrogen. The octanol–water partition coefficient, log P, for salmeterol is 3.88, compared with 0.66 for albuterol. Salmeterol is approximately 50-fold more selective than albuterol for the β2-receptor. Substantial evidence indicates that its long duration of action results from a specific binding interaction (“anchoring”) of the phenyl group at the end of the extended lipophilic side chain with a specific region of the β2-receptor, affording salmeterol a unique binding mechanism.
Pharmacokinetics
Salmeterol is 94 to 98% bound to human plasma proteins, both albumin and α1-acid glycoprotein, in vitro over the concentration range of 8 to 7722μg of base/L, much higher concentrations than those achieved following therapeutic doses of this drug. When salmeterol is inhaled, plasma concentrations of the drug often cannot be detected, even 30 minutes after administration of therapeutic doses. Larger inhaled doses give approximately proportionally increased blood concentrations. Plasma salmeterol concentrations of 0.1 to 0.2 and 1 to 2 μg/L were attained in healthy volunteers about 5 to 15 minutes after inhalation of single doses of 50 and 400μg, respectively. These concentrations were most probably related to the drug passing rapidly through the alveolar-capillary interface and probably into mucosal and submucosal vessels. In patients who inhaled salmeterol 50μg twice a day for 10 months, a second Cmax of 0.07 to 0.2 μg/L occurred 45 to 90 minutes after inhalation, probably due to gastrointestinal absorption of the swallowed drug (most of the dose delivered by a metered-dose inhaler is swallowed). Oral administration of 1mg of radiolabelled [14C]salmeterol (as salmeterol xinafoate) to two healthy individuals gave peak plasma salmeterol concentrations of about 650 μg/L at about 45 minutes. In another volunteer, t1 ?2β was about 5.5 hours. The dose-normalised Cmax values in comparative animal studies suggest that the systemic absorption and bioavailability in humans is greater (about 15 times greater) than that in rodents, being more closely related to that in dogs. Absorption may be high in humans, and first-pass metabolism less than in animals[1].
Clinical Use
Salmeterol usually is prescribed for severe persistent asthma following previous treatment with a short-acting β-agonist, such as albuterol. The noticeable differences between salmeterol and albuterol are the onset of action and their duration of action (Table 13.6). When used regularly every day as prescribed, inhaled albuterol decreases the number and severity of asthma attacks. It is not used, however, for relieving an asthma attack that has already started.
Side effects
Hoarseness, throat irritation, headache, rapid heartbeat, nervousness, cough, dry mouth/throat, or upset stomach may occur. To relieve dry mouth, suck on (sugarless) hard candy or ice chips, chew (sugarless) gum, drink water, or use a saliva substitute. This medication may raise your blood pressure. Rarely, this medication may cause sudden breathing problems/asthma right after you use it. If this occurs, use your quick-relief inhaler and get medical help right away.
Drug interactions
Many drugs besides salmeterol may affect the heart rhythm (QT prolongation), including amiodarone, dofetilide, pimozide, procainamide, quinidine, sotalol, macrolide antibiotics (such as erythromycin), among others. Before using salmeterol, report all medications you are currently using to your doctor or pharmacist.Other medications can affect the removal of salmeterol from your body, which may affect how salmeterol works. Examples include cobicistat, nefazodone, ritonavir, telithromycin, azole antifungals (such as ketoconazole, itraconazole), macrolide antibiotics (such as clarithromycin), HIV protease inhibitors (such as saquinavir), among others.
Metabolism
Salmeterol is metabolized predominantly through CYP3A4, an isoform of cytochrome P450. CYP3A4 is responsible for the aliphatic oxidation of the salmeterol base. Salmeterol is extensively metabolized by hydroxylation into alpha-hydroxy-salmeterol and subsequently eliminated through feces and urine. Salmeterol is 57.4% eliminated in the feces and 23% in the urine. At recommended doses, systemic concentrations of salmeterol are low or undetectable. Only at very high doses are blood concentrations increased.
References
[1] M. Cazzola, M. Matera, R. Testi. “Clinical Pharmacokinetics of Salmeterol.” Clinical Pharmacokinetics 41 1 (2002): 19–30.
Salmeterol Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| BIONNA MEDICINE CO.,LTD | 01056380788-8515; +8618518759099 | 790226113@qq.com | China | 52 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9350 | 55 |
| Lianyungang happen teng technology co., LTD | 15950718863 | wang666xt@163.com | CHINA | 295 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| HubeiwidelychemicaltechnologyCo.,Ltd | 18627774460 | faith@widelychemical.com | CHINA | 742 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9641 | 58 |
| WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD | +8615377521700 | wangwendy93@gmail.com | China | 868 | 58 |
Related articles
- Salmeterol: Mechanism of Persistence and Clinical Applications
- Salmeterol's mechanism involves debated factors like lipophilicity and receptor rebinding. It benefits asthma/COPD patients by....
- Mar 25,2024
- Salmeterol: pharmacological properties, therapeutic efficacy and safety
- Salmeterol can improve asthma control and lung functionby causing bronchodilation and inhibiting inflammatory responses in the....
- Oct 13,2023
- Uses and Side Effects of Salmeterol
- Salmeterol is used as a long-term (maintenance) treatment to prevent or decrease wheezing and trouble breathing caused by asth....
- Aug 25,2020
View Lastest Price from Salmeterol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-16 | Salmeterol
89365-50-4
|
US $0.00 / KG | 100g | 98%+ | 100kg | WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD | |
 |
2024-01-02 | Salmeterol
89365-50-4
|
US $55.00-550.00 / kg | 10kg | 0.99 | 20tons | Zibo Hangyu Biotechnology Development Co., Ltd | |
 |
2023-03-25 | Salmeterol
89365-50-4
|
US $60.00 / kg | 1kg | 99% | 20 tons | Hebei Duling International Trade Co. LTD |
-

- Salmeterol
89365-50-4
- US $0.00 / KG
- 98%+
- WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD
-

- Salmeterol
89365-50-4
- US $55.00-550.00 / kg
- 0.99
- Zibo Hangyu Biotechnology Development Co., Ltd
-

- Salmeterol
89365-50-4
- US $60.00 / kg
- 99%
- Hebei Duling International Trade Co. LTD