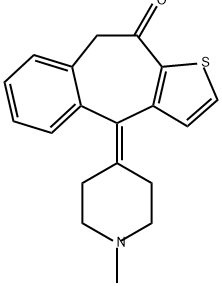
KETOTIFEN
- CAS No.
- 34580-13-7
- Chemical Name:
- KETOTIFEN
- Synonyms
- hc20-511;HC 20-511;KETOTIFEN;Ketotifeno;idinylidene)-;hiophen-10-one;Ketotifen base;KETOTIFENFUMARATC;Ketotifen Fumaratic;Ketotifen Free Base
- CBNumber:
- CB9249738
- Molecular Formula:
- C19H19NOS
- Molecular Weight:
- 309.43
- MDL Number:
- MFCD00599548
- MOL File:
- 34580-13-7.mol
- MSDS File:
- SDS
| Melting point | 152-153℃ |
|---|---|
| Boiling point | 488.9±45.0 °C(Predicted) |
| Density | 1.236 |
| storage temp. | Amber Vial, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly) |
| pka | 8.84±0.20(Predicted) |
| form | Solid |
| color | Light Yellow to Orange |
| InChI | InChI=1S/C19H19NOS/c1-20-9-6-13(7-10-20)18-15-5-3-2-4-14(15)12-17(21)19-16(18)8-11-22-19/h2-5,8,11H,6-7,9-10,12H2,1H3 |
| InChIKey | ZCVMWBYGMWKGHF-UHFFFAOYSA-N |
| SMILES | C12C(=O)CC3=CC=CC=C3/C(=C3/CCN(C)CC/3)/C=1C=CS2 |
| EWG's Food Scores | 1 |
| FDA UNII | X49220T18G |
| ATC code | R06AX17,S01GX08 |
| EPA Substance Registry System | Ketotifen (34580-13-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
| HS Code | 29349990 |
KETOTIFEN price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | K315413 | Ketotifen | 34580-13-7 | 500mg | $780 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0003086 | KETOTIFEN 95.00% | 34580-13-7 | 1G | $1347.37 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0003086 | KETOTIFEN 95.00% | 34580-13-7 | 5MG | $550 | 2021-12-16 | Buy |
KETOTIFEN Chemical Properties,Uses,Production
Originator
Zaditen,Wander,Switz.,1978
Uses
Anti-asthmatic.
Uses
Ketotifen is an intermediate in the synthesis of Ketotifen N-Oxide Hydrochloride (K315410), which is a metabolite of Ketotifen (K315100) in humans; formed by human liver microsome. For the free base, see K315115.
Definition
ChEBI: Ketotifen is an organic heterotricyclic compound that is 4,9-dihydro-10H-benzo[4,5]cyclohepta[1,2-b]thiophen-10-one which is substituted at position 4 by a 1-methylpiperidin-4-ylidene group. A blocker of histamine H1 receptors with a stabilising action on mast cells, it is used (usually as its hydrogen fumarate salt) for the treatment of asthma, where it may take several weeks to exert its full effect. It has a role as a H1-receptor antagonist and an anti-asthmatic drug. It is an organosulfur heterocyclic compound, an organic heterotricyclic compound, a cyclic ketone, a member of piperidines, an olefinic compound and a tertiary amino compound. It is a conjugate base of a ketotifen(1+).
Manufacturing Process
3.07 g of iodine-activated magnesium shavings are covered with a layer of 25
cc of tetrahydrofuran, and approximately 1/10 of a solution of 17.7 g of 4-
chloro-1-methylpiperidine base in 70 cc of absolute tetrahydrofuran is added.
The Grignard reaction is initiated by the addition of a few drops of 1,2-
dibromoethane. The remaining 4-chloro-1-methylpiperidine solution is then
added dropwise to the magnesium at such a rate that the reaction mixture
boils continuously at reflux without external heating. Boiling at reflux is then
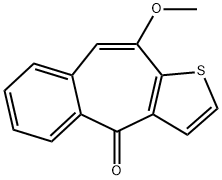
continued for 1 hour. 15.3 g of 10-methoxy-4H-benzo[4,5]cyclohepta[1,2-
b]thiophen-4-one are subsequently added portionwise at 20°C, within 40
minutes, with slight cooling. After stirring at 20°C for 1,5 hours, the reaction
solution is poured on a mixture of 180 g of ice and 20 g of ammonium
chloride. The free base is extracted with chloroform.
The chloroform solution is concentrated and the residue recrystallized from
270 cc of absolute ethanol. The pure 10-methoxy-4-(1-methyl-4-piperidyl)-
4H-benzo[4,5]cyclohepta[1,2-b]thiophen-4-ol base, having a melting point of
194°C to 196°C, is obtained in this manner. Microanalysis corresponds with
the formula C20H23NO2S.
A mixture of 3.4 g of 10-methoxy-4-(1-methyl-4-piperidyl)-4H-benzo[4,5]
cyclohepta [1,2-b]thiophen-4-ol base and 40 cc of 3N hydrochloric acid is kept
in a boiling water bath at 95°C to 100°C for 1 hour. The mixture is
subsequently made alkaline with concentrated caustic soda solution at 20°C
while cooling, and the free base is extracted with chloroform. The chloroform
solution is concentrated, and the residue is recrystallized from ethanol/water
1:1. The pure 4-(1-methyl-4-piperidylidene)-4H-benzo[4,5]cyclohepta [1,2-b]
thiophen-10(9H)-one base, having a melting point of 152°C to 153°C, is
obtained in this manner.
brand name
Zaditor (Novartis).
Therapeutic Function
Anti-asthmatic, Antihistaminic
General Description
Crystals (from ethyl acetate).
Fire Hazard
Flash point data for KETOTIFEN are not available. KETOTIFEN is probably combustible.
Clinical Use
Ketotifen is a potent, selective H1 antihistamine that also prevents release of transmitters from mast cells. It is approved in the United States for topical use to prevent itching of the eye because of allergic conjunctivitis, It is used as a systemic antiallergy agent in several countries outside the United States for the treatment of seasonal allergic rhinitis, hay fever, and asthma. Being structurally analogous to the cyproheptadine-like antihistamines, differences in activity of the two enantiomers (atropisomers) has been noted, being approximately six- to seven-fold in ligand displacement and rodent-based assays. Ketotifen has been shown to stabilize mast cells and to inhibit degranulation of eosinophils. Like olopatadine, it has been shown to interact with model membranes, stabilizing them by interaction with phospholipids monolayers.
KETOTIFEN Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| QINGDAO HONG JIN CHEMCIAL CO.,LTD. | 532-83657313 | hjt@hong-jin.com | China | 228 | 58 |
| Wuhan Cell Pharmaceutical Co., Ltd | +86-13129979210 +86-13129979210 | sales@cellwh.com | China | 376 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| SHANGHAI T&W PHARMACEUTICAL CO., LTD. | +86-021-61551413 +8618813727289 | contact@trustwe.com | China | 5738 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 | dominicguo@gk-bio.com | CHINA | 9427 | 58 |
| Alfa Chemistry | +1-5166625404 | Info@alfa-chemistry.com | United States | 21317 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49739 | 58 |
View Lastest Price from KETOTIFEN manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-10 | Ketotifen
34580-13-7
|
US $420.00-200.00 / Kg/Bag | 1Kg/Bag | 0.99 | 20 tons | Sinoway Industrial co., ltd. | |
 |
2023-10-10 | Ketotifen
34580-13-7
|
US $5.50-3.50 / g | 10g | 99% | 100kg | Wuhan Cell Pharmaceutical Co., Ltd | |
![10-(1-methylpiperidin-4-ylidene)-5H-benzo[1,2]cyclohepta[3,4-b]thiophen-4-one pictures](https://img.chemicalbook.com/ProductImageEN/2020-2/Small/f2c04b4e-a395-458a-b2a5-682c7b7d3454.png) |
2020-02-13 | 10-(1-methylpiperidin-4-ylidene)-5H-benzo[1,2]cyclohepta[3,4-b]thiophen-4-one
34580-13-7
|
US $1.00 / KG | 1KG | Min98% HPLC | g/kg/ton | Career Henan Chemical Co |
-

- Ketotifen
34580-13-7
- US $420.00-200.00 / Kg/Bag
- 0.99
- Sinoway Industrial co., ltd.
-

- Ketotifen
34580-13-7
- US $5.50-3.50 / g
- 99%
- Wuhan Cell Pharmaceutical Co., Ltd
-
![10-(1-methylpiperidin-4-ylidene)-5H-benzo[1,2]cyclohepta[3,4-b]thiophen-4-one pictures](https://img.chemicalbook.com/ProductImageEN/2020-2/Small/f2c04b4e-a395-458a-b2a5-682c7b7d3454.png)
- 10-(1-methylpiperidin-4-ylidene)-5H-benzo[1,2]cyclohepta[3,4-b]thiophen-4-one
34580-13-7
- US $1.00 / KG
- Min98% HPLC
- Career Henan Chemical Co
34580-13-7(KETOTIFEN)Related Search:
1of4