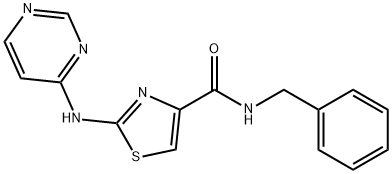
Thiazovivin
- CAS No.
- 1226056-71-8
- Chemical Name:
- Thiazovivin
- Synonyms
- TZV;CS-1925;Thiazovivin;Thiazovivin (Tzv);Thiazovivin, >=98%;Thiazovivin USP/EP/BP;N-Benzyl-2-(pyrimidin-4-ylamino)thiazole-4-carboxamide;N-Benzyl-2-(4-pyrimidinylamino)-1,3-thiazole-4-carboxamide;N-(Phenylmethyl)-2-(4-pyrimidinylamino)-4-thiazolecarboxamide;4-Thiazolecarboxamide, N-(phenylmethyl)-2-(4-pyrimidinylamino)-
- CBNumber:
- CB92512070
- Molecular Formula:
- C15H13N5OS
- Molecular Weight:
- 311.36
- MDL Number:
- MFCD16495823
- MOL File:
- 1226056-71-8.mol
- MSDS File:
- SDS
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P280-P305+P351+P338 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22 | |||||||||
| WGK Germany | 3 | |||||||||
| HS Code | 2934100090 | |||||||||
| NFPA 704 |
|
Thiazovivin price More Price(36)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML1045 | Thiazovivin ≥98% (HPLC) | 1226056-71-8 | 5mg | $149 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML1045 | Thiazovivin ≥98% (HPLC) | 1226056-71-8 | 25mg | $476 | 2024-03-01 | Buy |
| Cayman Chemical | 14245 | Thiazovivin ≥98% | 1226056-71-8 | 1mg | $40 | 2024-03-01 | Buy |
| Cayman Chemical | 14245 | Thiazovivin ≥98% | 1226056-71-8 | 5mg | $79 | 2024-03-01 | Buy |
| Cayman Chemical | 14245 | Thiazovivin ≥98% | 1226056-71-8 | 10mg | $139 | 2024-03-01 | Buy |
Thiazovivin Chemical Properties,Uses,Production
Description
Thiazovivin is a novel ROCK inhibitor with IC50 of 0.5 μM in a cell-free assay, promotes hESC survival after single-cell dissociation.
In vitro
Although displaying little impact on cell proliferation, Thiazovivin treatment significantly enhances the survival of human embryonic stem cells (hESCs) after enzymatic dissociation more than 30-fold, while homogenously maintaining pluripotency with the characteristic colony morphology, expression of typical pluripotency markers such as alkaline phosphatase (ALP), and normal karyotype. Dissociated hESCs treated with Thiazovivin display dramatically increased adhesion to matrigel-or laminin-coated plates but not to gelatin-coated plates within a few hours. Thiazovivin treatment increases cell-ECM adhesion-mediated β1 integrin activity, which synergizes with growth factors to promote cell survival. In addition to activating integrin, Thiazovivin but not Tyrintegin (Ptn) protects hESCs from death in the absence of ECM in suspension through E-cadherin-mediated cell-cell interaction. Thiazovivin treatment potently inhibits endocytosis of E-cadherin, consequently stabilizing E-cadherin on the cell surface and leading to reestablishment of cell-cell interaction, which is essential for hESC survival in ECM-free conditions. Thiazovivin but not Tyrintegin (Ptn) at 2 μM inhibits Rho-associated kinase (ROCK) activity and protects hESCs at a similar level as the widely used selective ROCK inhibitor Y-27632 at 10 μM, suggesting that Rho-ROCK signaling regulates cell-ECM and cell-cell adhesion. [1] Thiazovivin at 1 μM increases the reprogramming efficiency of CB mononuclear cells to induced pluripotent stem cells (iPSCs) by more than 10 times.
Description
Thiazovivin (1226056-71-8) dramatically improves (200-fold) the efficiency of induced pluripotent stem cell generation from human fibroblasts.1 Induces direct conversion of porcine embryonic fibroblasts into adipocytes.2?Cell permeable.
Uses
A compound that improves the survival of human embryonic stem cells (hESCs) upon trypsinization. In combination with ALK5 (TGFβ receptor) inhibitor SB-431542 and MEK inhibitor PD-0325901 (P217450), Thiazovivin promotes the transformation of fibroblasts into stem cells with a 200-fold efficiency over the classic method
Uses
Thiazovivin has been used in the generation of induced pluripotent stem cells (iPSCs) and induced neural stem cells (iNSCs) from human urine cells. It has also been used to study the the effect of pro-fibrotic inhibition on cardiac reprogramming.
Biochem/physiol Actions
Thiazovivin is an inhibitor of Rho associated coiled-coil containing protein kinase (ROCK). In vitro studies prove that thiazovivin is efficient in stimulating better morphology, expression of ionic transporter and protein involved in cell adhesion.
storage
-20°C
References
1) Lin et al. (2009), A chemical platform for improved induction of iPSC; Nature Methods, 6 805 2) Zhu et al. (2012), Direct conversion of porcine embryonic fibroblasts into adipocytes by chemical molecules; Cell Reprogram., 14 99
Thiazovivin Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 967 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29627 | 58 |
View Lastest Price from Thiazovivin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2019-07-06 | Thiazovivin
1226056-71-8
|
US $2.00 / KG | 1KG | 99% | customise | Career Henan Chemical Co |
-

- Thiazovivin
1226056-71-8
- US $2.00 / KG
- 99%
- Career Henan Chemical Co