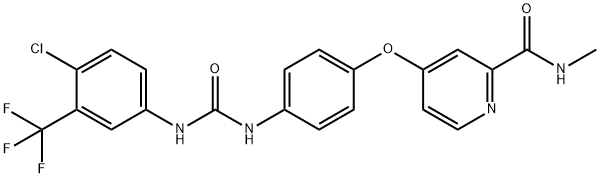
Sorafenib
- CAS No.
- 284461-73-0
- Chemical Name:
- Sorafenib
- Synonyms
- sorafenib;Nexavar;SORAFENIB-D3;BAY 43-9006;Sorafenib Free Base;4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)phenoxy)-N-methylpicolinamide;N-[4-Chloro-3-(trifluoromethyl)phenyl]-({4-[2-(N-methyl-carbamoyl)(4-pyridyloxy)]phenyl}amino)-carboxamide;SL-API;Sorafini;Forafenib
- CBNumber:
- CB9252510
- Molecular Formula:
- C21H16ClF3N4O3
- Molecular Weight:
- 464.82
- MDL Number:
- MFCD06411450
- MOL File:
- 284461-73-0.mol
- MSDS File:
- SDS
| Melting point | 202-204°C |
|---|---|
| Boiling point | 523.3±50.0 °C(Predicted) |
| Density | 1.454±0.06 g/cm3(Predicted) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Chloroform (Slightly), DMSO (Slightly) |
| pka | 12.89±0.70(Predicted) |
| form | White to off-white solid. |
| color | White to Off-White |
| Water Solubility | 100μg/L at 20℃ |
| LogP | 3.3 at 25℃ |
| CAS DataBase Reference | 284461-73-0(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | BAY 43-9006; Nexavar |
| FDA UNII | 9ZOQ3TZI87 |
| NCI Drug Dictionary | Nexavar |
| ATC code | L01EX02 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P280-P305+P351+P338 |
| Risk Statements | 68/20/21/22-37/38 |
| Safety Statements | 36-37-39 |
| HS Code | 29350090 |
Sorafenib price More Price(52)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML2653 | Sorafenib ≥98% (HPLC) | 284461-73-0 | 5MG | $48.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML2653 | Sorafenib ≥98% (HPLC) | 284461-73-0 | 25MG | $197 | 2024-03-01 | Buy |
| Cayman Chemical | 10009644 | BAY 43-9006 ≥98% | 284461-73-0 | 5mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 10009644 | Sorafenib ≥98% | 284461-73-0 | 25mg | $109 | 2024-03-01 | Buy |
| Cayman Chemical | 10009644 | BAY 43-9006 ≥98% | 284461-73-0 | 1mg | $25 | 2021-12-16 | Buy |
Sorafenib Chemical Properties,Uses,Production
Uses
Sorafenib, an orally active potent multi-kinases inhibitor,was approved in the U.S. for the treatment of advanced renalcell carcinoma. The drug targets both tumor cell proliferationand tumor angiogenesis kinases that include RAF,VEGFR-2, VEGFR-3, PDGFR-b, KIT and FLT-3. Sorafenibis being jointly developed by Bayer and Onyx in phase IIItrials as a single agent for the treatment of advanced hepato-cellular carcinoma and in combination with carboplatin andpaclitaxel in patients with advanced metastatic melanoma.Phase II trials in combination with doxorubicin for thetreatment of advanced hepatocellular carcinoma are alsounder investigation. Additional phase II trials are ongoingfor non-small cell lung cancer (NSCLC) and in postmenopausalwomen with estrogen receptor and/or progesteronereceptor-positive metastatic breast cancer. In addition, theNational Cancer Institute (NCI) is evaluating the compoundboth as a single therapy agent and in combination with otheroncology agents in phase II trials for several cancer indications.
Overview
Sorafenib tosylate is the tosylate form of sorafenib, which is a drug approved for the treatment of hepatocellular carcinoma and the treatment of advanced renal cell carcinoma (primary kidney cancer). Sorafenib is an oral receptor tyrosine kinase inhibitor that inhibits Raf serine/threonine kinases and receptor tyrosine kinases (vascular endothelial growth factor receptors 1, 2, 3 and platelet-derived growth factor-b, Flt-3 and c-kit) that are implicated in tumorigenesis and tumor progression.
Indications
It is indicated for the treatment of hepatocellular carcinoma and the treatment of advanced renal cell carcinoma (primary kidney cancer).
Mechanism of action
The bi-aryl urea sorafenib is an oral multikinase inhibitor that inhibits both cell surface tyrosine kinase receptors and downstream intracellular serine/threonine kinases in the Ras/MAPK cascade.[2-4] Receptor tyrosine kinases inhibited by sorafenib include vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2, VEGFR-3, platelet-derived growth factor receptor (PDGFR)-b, c-KIT, FMS-like tyrosine kinase 3 (FLT-3) and RET. Intracellular Raf serine/threonine kinase isoforms inhibited by sorafenib include Raf-1 (or C-Raf), wild-type B-Raf and mutant B-Raf.[3, 4] These kinases are involved in tumour cell proliferation and tumour angiogenesis.[3, 4]
The antiproliferative activity of sorafenib is variable in different tumor types and largely depends on the oncogenic signaling pathways that mediate tumor proliferation. Sorafenib has also been shown to induce apoptosis in several tumor cell lines. Although the mechanism through which sorafenib induces apoptosis is not fully elucidated and may vary between cell lines, a commonly observed theme is the inhibition of phosphorylation of the initiation factor eIF4E and loss of the antiapoptotic protein myeloid cell leukemia-1 (MCL-1)[5]. Recently, sorafenib was shown to inhibit hepatitis C viral replication in vitro[6], and in vitro studies have also shown some direct effects on immune cells [7]. Whether these effects
Side effects
The most common adverse reactions (20%), considered to be related to sorafenib, in patients with HCC or RCC are fatigue, weight loss, rash/desquamation, hand-foot skin reaction, alopecia, diarrhea, anorexia, nausea and abdominal pain [12].
Less frequent side effects (> 1 -10) include cardiac ischemia/infarction (3%), flushing, headache (10%), depression, fever, acne, exfoliative dermatitis, decreased appetite, dyspepsia, dysphagia, esophageal varices bleeding (2%), glossodynia, mucositis, stomatitis, xerostomia, erectile dysfunction, anemia, transaminases increased (transient), joint pain (10%), arthralgia, myalgia, hoarseness and flu-like syndrome.
Rare (< 1%) side effects of sorafenib include acute renal failure, alkaline phosphatase increased, arrhythmia, bilirubin increased, bone pain, cardiac failure, cerebral hemorrhage, congestive heart failure, dehydration, eczema, epistaxis, erythema multiforme, folliculitis, gastritis, gastrointestinal hemorrhage, gastrointestinal perforation, gastrointestinal reflux, gynecomastia, hypersensitivity (skin reaction, urticaria), hypertensive crisis, hyponatremia, hypothyroidism, infection, jaundice, myocardial infarction (MI), mouth pain, myocardial ischemia, pancreatitis, pleural effusion, preeclampsialike syndrome (reversible hypertension and proteinuria), renal failure, respiratory hemorrhage, reversible posterior leukoencephalopathy syndrome (RPLS), rhinorrhea, skin cancer (squamous cell/keratoacanthomas), thromboembolism, tinnitus, transient ischemic attack, tumor lysis syndrome, tumor pain and voice alteration.
References
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007 Jun; 132 (7): 2557-76
- Adnane L, Trail PA, Taylor I, et al. Sorafenib (BAY 439006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol 2005; 407: 597-612
- Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 2006 Oct; 5 (10): 835-44
- Wilhelm SM, Carter C, Tang LY, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004 Oct 1; 64 (19): 7099-109
- Yu C, Bruzek LM, Meng XW, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene 2005;24:6861-9
- Himmelsbach K, Sauter D, Baumert TF, et al. New aspects of an anti-tumour drug: sorafenib efficiently inhibits HCV replication. Gut 2009;58:1644-53
- Molhoek KR, McSkimming CC, Olson WC, et al. Apoptosis of CD4(+) CD25(high) T cells in response to Sirolimus requires activation of T cell receptor and is modulated by IL-2. Cancer Immunol Immunother 2009;58:867-76
- Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol 2005;23:965-72
- Clinical Pharmacology and Biopharmaceutics NDA Review for Sorafenib Tosylate (NDA 21 923), F.C.F.D.E.A. RESEARCH, Editor, 2005
- BAY 43-9006 (sorafenib) Investigator’s Brochure. Bayer Healthcare AG,Version 10.0, July 1, 2009
- European Medicines Agency. Sorafenib (Nexavar): summary of product characteristics [online].
- Blanchet B, Billemont B, Barete S, et al. Toxicity of sorafenib: clinical and molecular aspects. Expert Opin Drug Saf 2010;9:275-87
- https://www.drugs.com/cdi/sorafenib.html
Description
Sorafenib is a small molecular inhibitor of several kinases involved in tumor angiogenesis and proliferation, including, but not limited to, Raf (IC50=12nM for Raf-1), VEGFR (IC50=90nM for VEGFR-2 and IC50=12nM for VEGFR-3), and platelet derived growth factor receptor (IC50=57nM for PDGFR-b). Specifically, sorafenib blocks tumor progression by inhibiting cellular proliferation that is dependent on activation of the MAPK pathway (Raf) and/or inhibiting tumor angiogenesis through VEGFR and/or PDGFR. While it may be effective in the treatment of a variety of tumors, the first approvable indication is for renal cell carcinoma. Overall, the drug appears to be well tolerated by the majority of patients at the 400 mg b.i.d. continuous dosing. As an inhibitor of multiple kinases vital for tumor progression, sorafenib may possess wide-spectrum antitumor properties and may emerge as an effective weapon against a variety of solid tumors.
Chemical Properties
Light Yellow Solid
Originator
Bayer/Onyx (Germany)
Uses
Multiple kinase inhibitor targeting both RAF kinase and receptor tyrosine kinases that promote angiogensis. Antineoplastic.
Uses
A potent RAF kinase inhibitor. Antineoplastic
Uses
Sorafenib Tosylate (Bay 43-9006, Nexavar) is a small molecular inhibitor of VEGFR, PDGFR, c-Raf and B-Raf with IC50s of 18 nM, 10 nM, 3 nM and 15 nM, respectively.
Definition
ChEBI: Sorafenib is a member of the class of phenylureas that is urea in which one of the nitrogens is substituted by a 4-chloro-3-trifluorophenyl group while the other is substituted by a phenyl group which, in turn, is substituted at the para position by a [2-(methylcarbamoyl)pyridin-4-yl]oxy group. It has a role as an antineoplastic agent, an EC 2.7.11.1 (non-specific serine/threonine protein kinase) inhibitor, a tyrosine kinase inhibitor, an angiogenesis inhibitor, an anticoronaviral agent and a ferroptosis inducer. It is a pyridinecarboxamide, a member of monochlorobenzenes, an aromatic ether, a member of (trifluoromethyl)benzenes and a member of phenylureas.
Indications
Sorafenib (Nexavar(R), Bayer) was the first approved inhibitor targeting the vascular endothelial growth factor (VEGF) family kinases, which include VEGFR1, VEGR2, and VEGFR3. Sorafenib was originally approved for the treatment of renal cell carcinoma (RCC) in 2005, hepatocellular carcinoma in 2007, and locally recurrent or metastatic thyroid carcinoma refractory to radioactive iodine treatment in 2013. Six other approved inhibitors with VEGFRs as the main targets are sunitinib (Sutent(R), Pfizer) for RCC, soft tissue sarcoma, thyroid cancer,metastatic pancreatic tumors, gastrointestinal stromal tumor, and several other types of carcinomas; pazopanib (Votrient(R), GlaxoSmithKline) for RCC, soft tissue sarcoma, and thyroid cancer; axitinib (Inlyta(R), Pfizer) for RCC,thyroid cancer, and aplastic anemia, as well as T315I-mutant Bcr–Abl1-driven leukemia; regorafenib (Stivarga(R), Bayer) for gastrointestinal stromal tumors and colorectal cancer; nintedanib (Ofev(R), Boehringer Ingelheim) for the non-oncological indication of idiopathic pulmonary fibrosis; and lenvatinib (Lenvima(R), Eisai Inc.) for RCC and different types of thyroid cancers. Sunitinib, pazopanib, and lenvatinib bind to the “DFG-in”conformation of VEGFRs, while axitinib, regorafenib, and nintedanib bind to inactive VEGFRs adopting the “DFG-out”conformation.
brand name
Nexavar (Bayer HealthCare); Xarelto (Bayer HealthCare).
Flammability and Explosibility
Non flammable
Clinical Use
Protein kinase inhibitor:
Treatment of advanced renal cell carcinoma
Treatment of hepatocellular carcinoma
Treatment of thyroid cancer
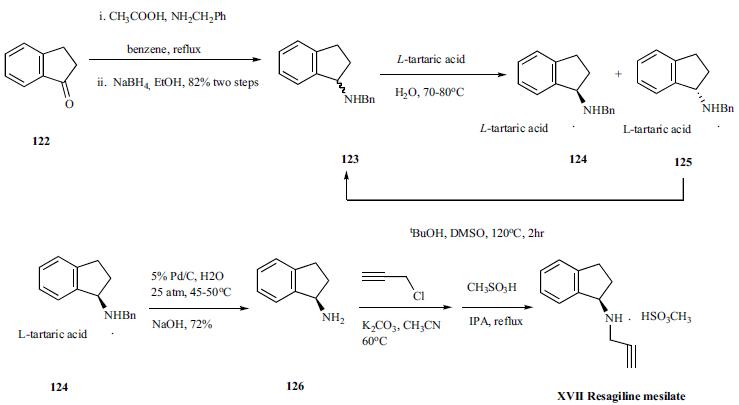
Synthesis
An improved, four-step synthesis in 63% overall yield
was published recently and is illustrated in the scheme.
Picolinic acid (127) was heated with Vilsmeier reagent for
16 hr to give 128 in 89% yield as an off-white solid. The
acid chloride 128 was treated with methylamine in methanol
at low temperature to give amide 129 in 88% yield as paleyellow
crystals after its crystallization from ethyl acetate.
4-Aminophenol anion was generated under a basic condition
and compound 129 was added to the anion solution to give
corresponding addition compound 131 in 87% yield. For an
unknown reason, potassium carbonate used in the reaction
increased the reaction rate significantly. Finally, compound
131 was condensed with isocyanate 132 in methylene chloride
to give sorafenib (XVIII) in 92% yield as a white solid.
Drug interactions
Potentially hazardous interactions with other drugs
Anticoagulants: may enhance effect of coumarins.
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis).
Antivirals: avoid with boceprevir.
Metabolism
Sorafenib is metabolised primarily in the liver and undergoes oxidative metabolism mediated by CYP3A4, as well as glucuronidation mediated by UGT1A9. 8 metabolites have been identified, during in vitro studies one has been shown to have equal activity to sorafenib. About 96% of a dose is excreted within 14 days, with 77%, mostly as unchanged drug, recovered in the faeces, and 19% in the urine as glucuronidated metabolites.
storage
Store at -20°C
Sorafenib Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 | dominicguo@gk-bio.com | CHINA | 9427 | 58 |
| Yangzhou Qinyuan Pharmatech Co.,ltd | +86-18752526868 | jennysun@yzqyyykj.com | China | 64 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 | sales@frappschem.com | China | 885 | 50 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9352 | 55 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 | bruce@xrdchem.cn | CHINA | 566 | 55 |
Related articles
- The brief introduction of Sorafenib
- Sorafenib is a multikinase inhibitor that has recently obtained Food and Drug Administration (FDA) approval for the treatment ....
- Apr 1,2024
- Sorafenib: A kinase inhibitor
- Sorafenib is a medicine that targets proteins in cancer cells and stops the cancer cells from growing. It is used to treat liv....
- Feb 15,2023
View Lastest Price from Sorafenib manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-12-26 | Sorafenib
284461-73-0
|
US $0.00-0.00 / kg | 1kg | 99%,single impurity<0.1 | 1 ton | Nanjing Fred Technology Co., Ltd | |
 |
2023-11-27 | Sorafenib tosylate
284461-73-0
|
US $0.00 / KG | 1KG | 99% | 20 TONS | Wuhan Senwayer Century Chemical Co.,Ltd | |
 |
2023-08-29 | Sorafenib tosylate
284461-73-0
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |
-

- Sorafenib
284461-73-0
- US $0.00-0.00 / kg
- 99%,single impurity<0.1
- Nanjing Fred Technology Co., Ltd
-

- Sorafenib tosylate
284461-73-0
- US $0.00 / KG
- 99%
- Wuhan Senwayer Century Chemical Co.,Ltd
-

- Sorafenib tosylate
284461-73-0
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd