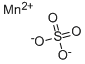Manganese sulfate
- CAS No.
- 7785-87-7
- Chemical Name:
- Manganese sulfate
- Synonyms
- MANGANESE SULFATE;MANGANESE SULFATE;MANGANESE SULPHATE;15:0-18:1-d7-PI;MANGANOUS(II)SULPHATE;Manganous sulphate, anhydrous;C039798;man-gro;ncic61143;anese suL
- CBNumber:
- CB9255526
- Molecular Formula:
- MnO4S
- Molecular Weight:
- 151
- MDL Number:
- MFCD00003464
- MOL File:
- 7785-87-7.mol
- MSDS File:
- SDS
| Melting point | 700°C |
|---|---|
| Boiling point | decomposes at 850℃ [HAW93] |
| Density | 3.250 |
| vapor pressure | 0Pa at 20℃ |
| form | white orthorhombic crystals |
| color | white orthorhombic crystals, crystalline |
| Water Solubility | g/100g solution H2O: 34.6 (0°C), 39.2 (25°C), 26.1 (100.7°C); solid phase, MnSO4 · 7H2O (0°C), MnSO4 ·H2O (25°C, 100.7°C) [KRU93] |
| LogP | -1.031 (est) |
| CAS DataBase Reference | 7785-87-7(CAS DataBase Reference) |
| SCOGS (Select Committee on GRAS Substances) | Manganous sulfate |
| FDA UNII | IGA15S9H40 |
| EPA Substance Registry System | Manganese(II) sulfate (7785-87-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H373-H411 | |||||||||
| Precautionary statements | P260-P314-P501 | |||||||||
| Hazard Codes | Xn,N | |||||||||
| Risk Statements | 48/20/22-51/53 | |||||||||
| Safety Statements | 22-61 | |||||||||
| RIDADR | UN 1760 | |||||||||
| NFPA 704 |
|
Manganese sulfate price
Manganese sulfate Chemical Properties,Uses,Production
Description
Manganese sulphate is the manganese salt of sulfate. It is an important precursor for the preparation of other manganese metal (e.g. manganese dioxide used in dry-cell batteries) and other chemical compounds. It is also an essential trace element which can be supplemented to the soils for plants as well as the feed for animals and livestock. It is also a useful trace element for medium of microbes. It can be manufactured through the reaction between manganese dioxide and sulfur dioxide or between potassium permanganate with sodium hydrogen sulfate and hydrogen peroxide.
Chemical Properties
Manganese sulfate ( MnSO4 ) is a pink crystalline solid. It occurs in nature as several mineral forms, jokokuite, pentahydrite, szmikite, and mallardite. It is used in industrial applications such as dyeing, porcelain glazing, and the manufacture of fertilizers and boiling oils.

In biochemistry, manganese is found in various superoxide dismutases. Manganese sulfate is used as a source of manganese ion in biological research, such as in culturing of Bacillus licheniformis and the induction of chromosomal abnormalities in plants. MnSO4 has been utilized to investigate the enzyme dependent glycosylation of endogenous glycoproteins in human skeletal muscle. A protocol that uses MnSO4 in the removal of contaminating genomic DNA in RNA samples for RTPCR has been published.
Uses
Manganese sulfate is used primarily as a fertilizer and as livestock supplement where soils are deficient in manganese, then in some glazes, varnishes, ceramics, and fungicides (EPA, 1984a; HSDB, 1997; Windholz, 1983).
Granular Manganese Sulfate is a granulated manganese fertiliser for dry application to the soil. It is also used as an ingredient in blended fertilisers.
Granular Manganese Sulfate is primarily intended for use in South Australia in planting fertilisers in crops grown on calcareous soils. Manganese deficiency most commonly occurs on alkaline (high pH) soils.
Manganese is quite abundant in the soil. Deficiency occurs because manganese is tied up or fixed in the soil in forms not available for plant uptake, i.e. at high pH, not because the soil is low in manganese.
Manganese applied as fertiliser can be rapidly converted to plant-unavailable forms.
For this reason, it is recommended that manganese be applied as foliar sprays where practicable, rather than to the soil. In horticultural crops, manganese can be applied with routine crop protection sprays.
Preparation
Manganese sulfate is made by reacting sulfuric acid with manganese dioxide.
MnO2 + H2SO4 → MnSO4
It can also be made by mixing potassium permanganate with sodium bisulfate and hydrogen peroxide.
References
[1]Blumberg, Ruth, and T. D. Morgan. "Chemical treatment of low-grade manganese ores: Conversion of manganese dioxide into manganese sulphate." Journal of Chemical Technology & Biotechnology Biotechnology 3.5(2010):223-233.
[2]Han, Xiao Zhao, et al. "Preparation of Manganese Sulfate from Pyrolusite by Abs orpting Sulfur Dioxide(Ⅰ)." Mining & Metallurgical Engineering (2003).
[3]Liu, Shi Liang, et al. "Effect of leaf surface spraying manganese sulfate on the trace element content and accumulation in alfalfa. " Acta Agrestia Sinica (2009).
[4]Sawhney, P. C, and N. D. Kehar. "Investigations on trace element-manganese. 2. Manganese content of common animal feeds as affected by maturity and irrigation. " Annals of Biochemistry & Experimental Medicine 21(1961):117.
[5]Kanner, Dov, and E. Schmell. "Process for production of recombinant human growth hormone using growth media with added trace elements from E. coli cells.", US9102970. 2015.
Description
A pale pink, granular, odorless powder. It is freely soluble in water and is insoluble in alcohol.
Chemical Properties
Translucent, pale-rose-red, efflorescent prisms. Soluble in water; insoluble in alcohol.
Uses
Supplement (trace mineral).
Definition
ChEBI: A metal sulfate in which the metal component is manganese in the +2 oxidation state.
Also known as manganous sulfate, MnS04,4H20 is water-soluble, translucent, efflorescent rose-red prisms which melt at 30°C. It is used in medicine,textile printing,and ceramics,as a fungicide and fertilizer, and in paint manufacture.
Safety Profile
Poison by intraperitoneal route. Questionable carcinogen with experimental neoplas tigenic data. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of SO2, so3, and Mn oxides. See also MANGANESE COMPOUNDS and SULFATES.
Synthesis
In the laboratory, manganese sulfate can be prepared by reacting manganese dioxide with sulfur dioxide. It can also be produced by mixing potassium permanganate with sodium bisulfate and hydrogen peroxide. Manganese sulfate is a by-product of several industrially significant oxidation reactions that utilize MnO2, including the production of hydroquinone and anisaldehyde.
Manganese sulfate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Kangcang new material Technology Co., LTD | +8619133911216 | Jany1001@kangcang.com.cn | China | 338 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-13102810335 | admin@hbsaisier.cn | China | 424 | 58 |
| Jiangsu Kolod Food Ingredients Co.,Ltd. | +86-518-85110578 +8618805133257 | sales3257@jskolod.com | China | 132 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 | info@longyupharma.com | China | 2531 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from Manganese sulfate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-19 | Manganese sulphate
7785-87-7
|
US $6.00 / KG | 1KG | More than 99% | 2000KG/Month | Hebei Saisier Technology Co., LTD | |
 |
2024-04-19 | Manganese sulphate
7785-87-7
|
US $9.00-70.00 / g | 10g | 99% | 10 tons | Hebei Kangcang new material Technology Co., LTD | |
 |
2023-12-25 | Manganese sulfate
7785-87-7
|
US $0.00 / kg | 1kg | 98% | 1000000 | Hebei Jingbo New Material Technology Co., Ltd |
-

- Manganese sulphate
7785-87-7
- US $6.00 / KG
- More than 99%
- Hebei Saisier Technology Co., LTD
-

- Manganese sulphate
7785-87-7
- US $9.00-70.00 / g
- 99%
- Hebei Kangcang new material Technology Co., LTD
-

- Manganese sulfate
7785-87-7
- US $0.00 / kg
- 98%
- Hebei Jingbo New Material Technology Co., Ltd
7785-87-7(Manganese sulfate)Related Search:
1of4