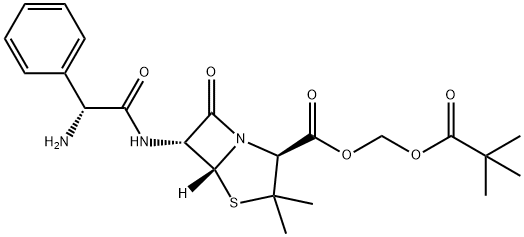
PIVAMPICILLIN
- CAS No.
- 33817-20-8
- Chemical Name:
- PIVAMPICILLIN
- Synonyms
- mk191;PIVAPICILLIN;PIVAMPICILLIN;pivampiclillin;Pivampicillin CRS;pivaloylampicillin;PIVAMPICILLIN USP/EP/BP;pivaloyloxymethylampicillinate;PIVAMPICILLIN EPP(CRM STANDARD);ampicillinpivaloyloxymethylester
- CBNumber:
- CB9362547
- Molecular Formula:
- C22H29N3O6S
- Molecular Weight:
- 463.55
- MDL Number:
- MFCD00869402
- MOL File:
- 33817-20-8.mol
| Boiling point | 679.0±55.0 °C(Predicted) |
|---|---|
| Density | 1.33±0.1 g/cm3(Predicted) |
| solubility | Practically insoluble in water, freely soluble in methanol, soluble in anhydrous ethanol. It dissolves in dilute acids. |
| pka | pKa 7.0 (Uncertain) |
| FDA UNII | 0HLM346LL7 |
| ATC code | J01CA02 |
PIVAMPICILLIN Chemical Properties,Uses,Production
Description
Pivampicillin caused sensitization in 56 workers at a penicillin factory. Pivampicillin and pivmecillinam were responsible for contact dermatitis in pharmaceutical production workers. Ampicillin, mecillinam, penicillin V and penicillin G were also implicated in cross reactions.
Chemical Properties
White or almost white, crystalline powder.
Originator
Maxifen ,Sharp and Dohme, W. Germany ,1972
Uses
Antibacterial.
Definition
ChEBI: Pivampicillin is a penicillanic acid ester that is the pivaloyloxymethyl ester of ampicillin. It is a prodrug of ampicillin. It has a role as a prodrug. It is a penicillanic acid ester and a pivaloyloxymethyl ester. It is functionally related to an ampicillin.
Manufacturing Process
(A) Pivaloyloxymethyl D(-)-α-azidobenzylpenicillinate: To a suspension of potassium D(-)α-azidobenzylpenicillinate (4.14 g) and potassium dicarbonate(1.5 g) in acetone (100 ml) and 10% aqueous sodium iodide (2 ml), chloromethyl pivalate (2.7 ml) was added and the mixture refluxed for 2 hours. After cooling, the suspension was filtered and the filtrate evaporated to dryness in vacuo. The remaining residue was washed repeatedly by decantation with petroleum ether to remove unreacted chloromethyl pivalate. The oily residue was taken up in ethyl acetate (100 ml), and the resulting solution washed with aqueous sodium bicarbonate and water, dried and evaporated in vacuo to yield the desired compound as a yellowish gum, which crystallized from ether, melting point 114°C to 115°C.
(B) Pivaloyloxymethyl D(-)-α-aminobenzylpenicillinate, hydrochloride: To a solution of pivaloyloxymethyl D(-)-α-azidobenzylpenicillinate (prepared as described above) in ethyl acetate (75 ml) a 0.2 M phosphate buffer (pH 2.2) (75 ml) and 10% palladium on carbon catalyst (4 g) were added, and the mixture was shaken in a hydrogen atmosphere for 2 hours at room temperature. The catalyst was filtered off, washed with ethyl acetate (25 ml) and phosphate buffer (25 ml), and the phases of the filtrate were separated. The aqueous phase was washed with ether, neutralized (pH 6.5 to 7.0) with aqueous sodium bicarbonate, and extracted with ethyl acetate (2 x 75 ml). To the combined extracts, water (75 ml) was added, and the pH adjusted to 2.5 with 1 N hydrochloric acid. The aqueous layer was separated, the organic phase extracted with water (25 ml), and the combined extracts were washed with ether, and freeze-dried. The desired compound was obtained as a colorless, amorphous powder.
The purity of the compound was determined iodometrically to be 91%. A crystalline hydrochloride was obtained from isopropanol with a melting point of 155°C to 156°C (dec.).
Therapeutic Function
Antibacterial
Contact allergens
Pivampicillin is a prodrug of ampicillin. It caused sensitization in 56 workers at a penicillin factory. Pivampicillin and pivmecillinam were responsible for contact dermatitis in pharmaceutical production workers. Ampicillin, mecillinam or amdinocillin, penicillin V and penicillin G were also implicated in cross-reactions.
PIVAMPICILLIN Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | 1057@dideu.com | China | 3684 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9456 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29321 | 58 |
| Guangzhou PI PI Biotech Inc | +86-020-81716320 +86-13602409664 | Sales@pipitech.com | China | 3635 | 55 |
| Beijing HuaMeiHuLiBiological Chemical | 010-56205725 | waley188@sohu.com | China | 12338 | 58 |
| Shanghai T&W Pharmaceutical Co., Ltd. | +86 21 61551611 | China | 9901 | 58 | |
| ChemStrong Scientific Co.,Ltd | 0755-66853366 13670046396 | sales@chem-strong.com | China | 17982 | 56 |
| Alta Scientific Co., Ltd. | 022-6537-8550 15522853686 | sales@altasci.com.cn | China | 4515 | 55 |
| BOC Sciences | 16314854226 | info@bocsci.com | United States | 9926 | 65 |
View Lastest Price from PIVAMPICILLIN manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-06-28 | PIVAMPICILLIN USP/EP/BP
33817-20-8
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited | |
 |
2020-05-12 | PivaMpicillin
33817-20-8
|
US $1.00 / KG | 1KG | 99% | 20T | Shaanxi Dideu Medichem Co. Ltd |
-

- PIVAMPICILLIN USP/EP/BP
33817-20-8
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- PivaMpicillin
33817-20-8
- US $1.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd