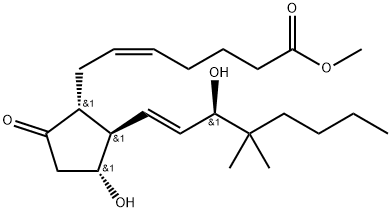
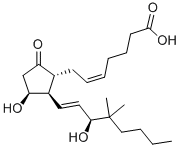
16,16-DIMETHYL PROSTAGLANDIN E2
- CAS No.
- 39746-25-3
- Chemical Name:
- 16,16-DIMETHYL PROSTAGLANDIN E2
- Synonyms
- 15r)-13e;opentyl)-;16-dimethyl PGE2;16,16-dimethyl-pge2;16,16-Dimethyl dinoprostone;QAOBBBBDJSWHMU-WMBBNPMCSA-N;Suppliedasayellow,viscousoil;16-Dimethyl prostaglandin E2;16,16-DimethylprostaglandineE2;16,16-DIMETHYL PROSTAGLANDIN E2
- CBNumber:
- CB9416741
- Molecular Formula:
- C22H36O5
- Molecular Weight:
- 380.52
- MDL Number:
- MFCD00151141
- MOL File:
- 39746-25-3.mol
| Flash point | -13℃ |
|---|---|
| storage temp. | -20°C |
| solubility | Soluble in methyl acetate |
| form | methyl acetate solution |
| FDA UNII | M790V82VAC |
| NCI Drug Dictionary | 16, 16-dimethyl prostaglandin E2 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H319-H336 | |||||||||
| Precautionary statements | P210-P305+P351+P338 | |||||||||
| Hazard Codes | F,Xn,Xi | |||||||||
| Risk Statements | 11-20/21/22-36/37/38-67-66-36 | |||||||||
| Safety Statements | 16-26-36 | |||||||||
| RIDADR | UN 1231 3/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| NFPA 704 |
|
16,16-DIMETHYL PROSTAGLANDIN E2 price More Price(10)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | D0160 | 16,16-Dimethylprostaglandin E2 methyl acetate solution | 39746-25-3 | 1mg | $348 | 2023-06-20 | Buy |
| Cayman Chemical | 14750 | 16,16-dimethyl Prostaglandin E2 ≥98% | 39746-25-3 | 1mg | $86 | 2024-03-01 | Buy |
| Cayman Chemical | 14750 | 16,16-dimethyl Prostaglandin E2 ≥98% | 39746-25-3 | 5mg | $399 | 2024-03-01 | Buy |
| Cayman Chemical | 14750 | 16,16-dimethyl Prostaglandin E2 ≥98% | 39746-25-3 | 10mg | $755 | 2024-03-01 | Buy |
| Tocris | 4027 | 16,16-Dimethylprostaglandin E2 ≥98%(HPLC) | 39746-25-3 | 1 | $125 | 2021-12-16 | Buy |
16,16-DIMETHYL PROSTAGLANDIN E2 Chemical Properties,Uses,Production
Description
16,16-dimethyl PGE2 is a competitive inhibitor of 15-
Definition
ChEBI: 16,16-dimethylprostaglandin E2 is a prostanoid that is prostaglandin E2 in which both of the hydrogens at position 16 have been replaced by methyl groups. A synthetic analogue of prostaglandin E2, it is a potent inhibitor of pancreatic function and growth of experimental tumors. It also protects the gastric mucosa, prevents ulceration, and promotes the healing of peptic ulcers. It has a role as a radiation protective agent, an anti-ulcer drug and a gastrointestinal drug. It is a prostanoid, a monocarboxylic acid, a secondary allylic alcohol and a member of cyclopentanones.
in vitro
dmpge2 was reported to cause an increase in runx11/cmyb1 hscs, while hscs were inhibited by indomethacin treatment in 90% of embryos. moreover, dmpge2 had minimal effects on the vasculature, while indomethacin altered the intersomitic vessels slightly. imaged by confocal microscopy, red-labelled hscs and endothelium embryos showed significantly increased hscs following dmpge2 exposure [1].
in vivo
in a heterotopic model of rat allograft rejection, dmpge2 could delay the rejection onset, but all animals developed severe rejection and died subsequently. treatment of animals with low-dose csa in combination with dmpge2 led to a delay in the onset as well as a reduction in the intensity of allograft rejection. in addition, a statistical relationship between procoagulant activity levels and the time of onset of rejection was observed [1].
storage
Store at -80°C
References
[1] north te,goessling w,walkley cr,lengerke c,kopani kr,lord am,weber gj,bowman tv,jang ih,grosser t,fitzgerald ga,daley gq,orkin sh,zon li. prostaglandin e2 regulates vertebrate haematopoietic stem cell homeostasis. nature.2007 jun 21;447(7147):1007-11.
[2] koh ih,kim pc,chung sw,waddell t,wong py,gorczynski r,levy ga,cohen z. the effects of 16, 16 dimethyl prostaglandin e2 therapy alone and in combination with low-dose cyclosporine on rat small intestinal transplantation. transplantation.1992 oct;54(4):592-8.
16,16-DIMETHYL PROSTAGLANDIN E2 Preparation Products And Raw materials
Raw materials
Preparation Products
16,16-DIMETHYL PROSTAGLANDIN E2 Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33349 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 | 3bsc@sina.com | China | 15848 | 69 |
| Sigma-Aldrich | 021-61415566 800-8193336 | orderCN@merckgroup.com | China | 51471 | 80 |
| EMMX Biotechnology LLC | 888-539-0666 | info@emmx.com | United States | 8449 | 60 |
| Shanghai EFE Biological Technology Co., Ltd. | 021-65675885 18964387627 | info@efebio.com | China | 9709 | 58 |
| Hangzhou Synstar pharmaceutical Technology CO.,Ltd | 0571-85361029 | synstar518@163.com | China | 1991 | 58 |
| ChemeGen(Shanghai) Biotechnology Co.,Ltd. | 18818260767 | sales@chemegen.com | China | 11289 | 58 |
| Energy Chemical | 021-58432009 400-005-6266 | marketing1@energy-chemical.com | China | 44894 | 58 |
39746-25-3(16,16-DIMETHYL PROSTAGLANDIN E2)Related Search:
1of3





