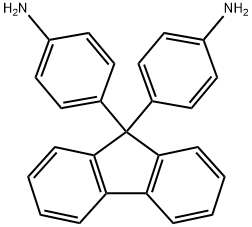
9,9-Bis(4-aminophenyl)fluorene
- CAS No.
- 15499-84-0
- Chemical Name:
- 9,9-Bis(4-aminophenyl)fluorene
- Synonyms
- FDA;BAFL;AKOS BBB/416;Zinc03897005;Anilinefluorene;BIO-FARMA BF003152;TIMTEC-BB SBB008629;9,9-bis(4-aminopheny;bisaminophenylfluorene;Bis(4-aminophenyl)fluorene
- CBNumber:
- CB9439319
- Molecular Formula:
- C25H20N2
- Molecular Weight:
- 348.44
- MDL Number:
- MFCD00039156
- MOL File:
- 15499-84-0.mol
- MSDS File:
- SDS
| Melting point | 237-239 °C (lit.) |
|---|---|
| Boiling point | 535.2±50.0 °C(Predicted) |
| Density | 1.245±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| Water Solubility | Insoluble in water |
| form | powder to crystal |
| pka | 4.92±0.10(Predicted) |
| color | White to Almost white |
| InChI | InChI=1S/C25H20N2/c26-19-13-9-17(10-14-19)25(18-11-15-20(27)16-12-18)23-7-3-1-5-21(23)22-6-2-4-8-24(22)25/h1-16H,26-27H2 |
| InChIKey | KIFDSGGWDIVQGN-UHFFFAOYSA-N |
| SMILES | C1(C2=CC=C(N)C=C2)(C2=CC=C(N)C=C2)C2=C(C=CC=C2)C2=C1C=CC=C2 |
| CAS DataBase Reference | 15499-84-0(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H312+H332-H319-H335 | |||||||||
| Precautionary statements | P261-P280-P301+P312-P302+P352+P312-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 20/21/22-36/37 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 3 | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29215900 | |||||||||
| NFPA 704 |
|
9,9-Bis(4-aminophenyl)fluorene price More Price(25)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 437913 | 4,4′-(9-Fluorenylidene)dianiline 99% | 15499-84-0 | 25g | $251 | 2024-03-01 | Buy |
| TCI Chemical | B1549 | 9,9-Bis(4-aminophenyl)fluorene >98.0%(T) | 15499-84-0 | 25g | $110 | 2024-03-01 | Buy |
| TCI Chemical | B1549 | 9,9-Bis(4-aminophenyl)fluorene >98.0%(T) | 15499-84-0 | 100g | $330 | 2024-03-01 | Buy |
| TCI Chemical | B2654 | 9,9-Bis(4-aminophenyl)fluorene (purified by sublimation) >99.0%(HPLC)(T) | 15499-84-0 | 1g | $91 | 2024-03-01 | Buy |
| TRC | F591593 | 4,4''-(9H-Fluorene-9,9-diyl)dianiline | 15499-84-0 | 2.5g | $90 | 2021-12-16 | Buy |
9,9-Bis(4-aminophenyl)fluorene Chemical Properties,Uses,Production
Description
9,9-Bis(4-aminophenyl)fluorene (FDA) is a fluorene derivative with two aminophenyl substituents on the 9-position. This compound has partial conjugation and can be utilized as a dopant-free organic hole-transporting material in inverted perovskite solar cells. Due to its diaminophenyl groups, FDA is commonly employed in synthesizing polyimides that possess high thermal and hydrolytic stability for optical fiber light guide coatings. Because the FDA is not fully aromatized, it is chosen to produce thermally stable transparent polyimides. Additionally, the polyimide derived from 9,9-bis(4-aminophenyl)fluorene can be further modified through Friedel-Crafts alkylation on the fluorene moiety, as the polyimide backbone remains chemically stable.
Chemical Properties
White powder
Uses
9,9-bis(4-aminophenyl)fluorene be used for preparation of high-strength material and high-temperature polymers.
Preparation
The synthesis of 9,9-Bis(4-aminophenyl)fluorene can be carried out through the following stepwise procedure:
Prepare the reaction mixture:
Place 3.6 g of fluorenone (20 mmol), 7.2 g of aniline hydrochloride (55.6 mmol), 0.5 g of sodium bisulfite (4.8 mmol), 8.8 mL of aniline (96.4 mmol), and 6 mL of toluene in a 100 mL two-necked flask.
Ensure the reaction is conducted under nitrogen protection.
Add a water separator to the two-necked flask.
Begin the reaction:
Stir the reaction mixture and slowly increase the temperature to 120 °C.
Allow the reaction to continue for 30 minutes.
Separate the green liquid produced in the water separator.
Further reaction at elevated temperature:
Continue the reaction at 120 °C for an additional 3 hours.
Separate the water produced in the water separator.
Increase temperature and maintain reaction:
Slowly raise the temperature to 135 °C.
Keep the reaction at this temperature for 1.5 hours.
Monitor for completion:
Check the water separator for any further production of water.
Cease the reaction when no more water is produced in the water separator.
Cool down and adjust pH:
Allow the reacted material to cool to around 60 ℃.
Add 10 wt% potassium hydroxide solution to the reaction mixture.
Adjust the pH of the mixture to 9.
Stir the solution for 15 minutes while it is still hot.
Filtration and purification:
Filter the solution to remove any solid impurities.
Wash the filter cake with water.
Recrystallize the washed filter cake using 30 mL of toluene.
Filter the recrystallized product to obtain pure rice-white product.
Yield determination:
Weigh the obtained pure product, which should amount to 6.55 g.
Calculate the yield by dividing the obtained mass by the theoretical maximum mass and multiplying by 100%.
The yield of the pure rice-white product should be 94%.
General Description
4,4′-(9-Fluorenylidene)dianiline is a diamine.
References
[1] PHAM H D, GIL-ESCRIG L, FERON K, et al. Boosting inverted perovskite solar cell performance by using 9,9-bis(4-diphenylaminophenyl)fluorene functionalized with triphenylamine as a dopant-free hole transporting material?[J]. Journal of Materials Chemistry A, 2019, 20: 12507-12517. DOI:10.1039/C9TA01681C.
[2] G. SONG C. C. Colorless, heat resistant polyimide films derived from 2,3,3′,4′-biphenyltetracarboxylic dianhydride[J]. IOP Conference Series: Materials Science and Engineering, 2020, 73 1. DOI:10.1088/1757-899X/733/1/012035.
[3] WEI J, YU L, YAN L, et al. Synthesis of 9,9-bis(4-hydroxyphenyl) fluorene catalyzed by bifunctional ionic liquids?[J]. RSC Advances, 2021, 52: 32559-32564. DOI:10.1039/D1RA05967J.
[4] SURASAK SEESUKPHRONRARAK. Synthesis and characterization of 9,9-bis(4-hydroxyphenyl and 4-aminophenyl)dibenzofluorenes: Novel fluorene-based monomers[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2019, 57 24: 2602-2605. DOI:10.1002/pola.29540.
[5] HOU CHIEN CHANG. Synthesis of 9,9-bis(4-aminophenyl)fluorene-based benzoxazine and properties of its high-performance thermoset[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2012, 50 11: 2201-2210. DOI:10.1002/pola.25993.
[6] Patent: CN107892649, 2018, A, . Location in patent: Paragraph 0023-0042
[7] Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 23, p. 240
[8] DONGHUI YU. Fluorene-Based Phosphine Oxide Host Materials for Blue Electrophosphorescence: An Effective Strategy for a High Triplet Energy Level[J]. Chemistry - A European Journal, 2011, 17 9: 2592-2596. DOI:10.1002/chem.201003434.
9,9-Bis(4-aminophenyl)fluorene Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Z&T Chemical Co., Ltd | +86-21-33561011 13818804961 | Sales@ztchemical.com.cn | China | 402 | 58 |
| Shanghai Daeyeon Chemicals Co., Ltd | 021-64478606 +8615900664856 | daeyeon001@vip.163.com | China | 2062 | 58 |
| Heynova (Shanghai) New Material Technology CO., Ltd | +86-17821320848 | zoe@heynovachem.com | China | 431 | 58 |
| CHINATECH(TIANJIN) CHEMICAL CO.,LTD. | +8618222493702 | sales8@chinatechem.com | China | 61 | 58 |
| Jiangsu Ever Galaxy Chemical Co.,Ltd | +8618602515822 | info@egchemical.com | China | 76 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7786 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
View Lastest Price from 9,9-Bis(4-aminophenyl)fluorene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-28 | 4,4'-(9H-fluorene-9,9-diyl)dianiline
15499-84-0
|
US $0.00 / kg | 25kg | 98%-99% | Inquiry | PNP Biotech Co. Ltd | |
 |
2024-01-08 | 9,9-Bis(4-aminophenyl)fluorene
15499-84-0
|
US $111.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-07-25 | 9,9-BIS(4-AMINOPHENYL)FLUORENE
15499-84-0
|
US $0.00 / kg | 1kg | 99% | 20 tons | Hebei Yanxi Chemical Co., Ltd. |
-

- 4,4'-(9H-fluorene-9,9-diyl)dianiline
15499-84-0
- US $0.00 / kg
- 98%-99%
- PNP Biotech Co. Ltd
-

- 9,9-Bis(4-aminophenyl)fluorene
15499-84-0
- US $111.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- 9,9-BIS(4-AMINOPHENYL)FLUORENE
15499-84-0
- US $0.00 / kg
- 99%
- Hebei Yanxi Chemical Co., Ltd.
15499-84-0(9,9-Bis(4-aminophenyl)fluorene)Related Search:
1of4