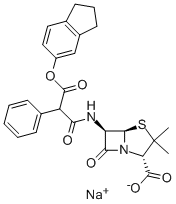
CARBENICILLIN INDANYL SODIUM
- CAS No.
- 26605-69-6
- Chemical Name:
- CARBENICILLIN INDANYL SODIUM
- Synonyms
- i-cbpc;C12712;GU-Pen;cp15464-2;geocillin;carindapen;carindacillin;carindacillinsodium;carbenicillinindanyl;carbenicillinindanylsodium
- CBNumber:
- CB9501393
- Molecular Formula:
- C26H26N2O6S.Na
- Molecular Weight:
- 0
- MDL Number:
- MOL File:
- 26605-69-6.mol
- MSDS File:
- SDS
| Melting point | 207-213° |
|---|---|
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Water (Slightly, Heated, Sonicated) |
| form | Solid |
| color | White to Off-White |
| Stability | Hygroscopic, Temperature Sensitive |
| FDA UNII | 4OUL81K2RT |
| ATC code | J01CA05 |
CARBENICILLIN INDANYL SODIUM price More Price(2)
CARBENICILLIN INDANYL SODIUM Chemical Properties,Uses,Production
Description
Carbenicillin indanyl was synthesized by Pfizer in 1972 as an orally active carbenicillin. It shows strong activity against a variety of bacteria in vitro, and, when administered orally, it behaves as carbenicillin after being hydrolyzed by intestinal esterase.
Originator
Geocillin,Roerig,US,1972
Uses
Carbenicillin indanyl sodium has been shown to reduce blood pressure in mammals and also has been used as a β-lactam antibiotic.
Uses
Has been shown to reduce blood pressure in mammals and also has been used as a β-lactam antibiotic.
Definition
ChEBI: Carindacillin sodium is an organic sodium salt. It contains a carindacillin(1-).
Manufacturing Process
(A) Preparation of Phenylchlorocarbonyl Ketene: To phenylmalonic acid (20 g)
in ethyl ether (100 ml) there is added phosphorus pentachloride (46 g). A
vigorous reaction occurs. The reaction mixture is refluxed for 4 hours then the
ether partially removed by heating on a steam bath. The reaction mixture
becomes black when about half the ether is removed and the remaining ether
is removed under reduced pressure (at 100 mm). The residue is distilled
under vacuum and the fraction boiling at 75° to 90°C at 1.5 to 4 mm
collected. The product, a yellow liquid, is redistilled at 74°C and 1.5 mm. It
shows a strong peak in the infrared region of the spectrum at 4.69 mu.
Repetition of this procedure but using 10 g of phenylmalonic acid instead of
20 g produces a less vigorous reaction on addition of the phosphorus
pentachloride. The same product is obtained.
(B) Acylation of 6-Aminopenicillanic Acid: To a solution of the aryl
halocarbonyl ketene (0.1 mol) in methylene chloride (sufficient to provide a
clear solution and generally from about 5 to 10 ml per gram of ketene) there
is added the proper alcohol R2OH (0.1 mol), in this case 5-indanyl alcohol.
The reaction mixture is maintained under an atmosphere of nitrogen and
stirred for a period of from 20 minutes to 3 hours, care being taken to exclude moisture. The temperature may range from about -70° to about -
20°C. The infrared spectrum of the mixture is then taken to determine and
confirm the presence of the ketene ester. A solution of 6-aminopenicillanic
acid-triethylamine salt (0.1 mol) in methylene chloride (50 ml) is added and
the mixture stirred at -70° to -20°C for 10 minutes. The cooling bath is then
removed and the reaction mixture stirred continuously and allowed to warm to
room temperature.
Various isolation methods are then spelled out in US Patent 3,679,801.
brand name
Geocillin (Pfizer).
Therapeutic Function
Antibacterial
Clinical Use
Efforts to obtain orally active forms of carbenicillin led to theeventual release of the 5-indanyl ester carbenicillin indanyl,6-[2-phenyl-2-(5-indanyloxycarbonyl)acetamido]penicillanicacid (Geocillin), in 1972. Approximately 40% of theusual oral dose of indanyl carbenicillin is absorbed. After absorption,the ester is hydrolyzed rapidly by plasma and tissueesterases to yield carbenicillin. Thus, although the highlylipophilic and highly protein-bound ester has in vitro activitycomparable with that of carbenicillin, its activity in vivo isdue to carbenicillin. Indanyl carbenicillin thus provides anorally active alternative for the treatment of carbenicillinsensitivesystemic and urinary tract infections caused byPseudomonas spp., indole-positive Proteus spp., and selectedspecies of Gram-negative bacilli.
Clinical trials with indanyl carbenicillin revealed a relativelyhigh frequency of GI symptoms (nausea, occasionalvomiting, and diarrhea). It seems doubtful that the highdoses required for the treatment of serious systemic infectionscould be tolerated by most patients. Indanyl carbenicillinoccurs as the sodium salt, an off-white, bitter powderthat is freely soluble in water. It is stable in acid. It should beprotected from moisture to prevent hydrolysis of the ester.
Veterinary Drugs and Treatments
Carbenicillin was used parenterally in the treatment of systemic Pseudomonas aeruginosa infections in small animals, usually in combination with an appropriate aminoglycoside agent, but in the USA the injectable is no longer available and most clinicians use ticarcillin or piperacillin in its place. Because the oral form is poorly absorbed and the drug has a rapid elimination half-life, oral therapy is only indicated for the treatment of susceptible urinary tract (and possibly prostate) infections as levels are too low in serum and other tissues for adequate therapy in other systemic Pseudomonas infections.
CARBENICILLIN INDANYL SODIUM Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | 1057@dideu.com | China | 3586 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9358 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29271 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| Alfa Chemistry | +1-5166625404 | Info@alfa-chemistry.com | United States | 21317 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| Sinopharm Chemical Reagent Co,Ltd. | 86-21-63210123 | sj_scrc@sinopharm.com | China | 9823 | 79 |
| Beijing Solarbio Science & Tecnology Co., Ltd. | 010-50973186 4009686088 | 3193328036@qq.com | China | 18352 | 68 |
| Shenzhen Polymeri Biochemical Technology Co., Ltd. | +86-400-002-6226 13028896684 | sales@rrkchem.com | China | 55717 | 58 |
| Energy Chemical | 021-58432009 400-005-6266 | marketing1@energy-chemical.com | China | 44894 | 58 |
View Lastest Price from CARBENICILLIN INDANYL SODIUM manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2020-05-12 | SodiuM Indanylcarbinicillin
26605-69-6
|
US $1.00 / KG | 1KG | 99% | 20T | Shaanxi Dideu Medichem Co. Ltd |
-

- SodiuM Indanylcarbinicillin
26605-69-6
- US $1.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd
26605-69-6(CARBENICILLIN INDANYL SODIUM)Related Search:
1of2