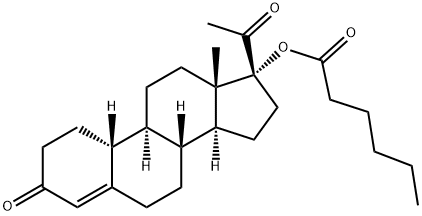
GESTONORONE CAPROATE
- CAS No.
- 1253-28-7
- Chemical Name:
- GESTONORONE CAPROATE
- Synonyms
- SH 582;ZK 5623;Depostat;SH 80582;SM-80582;NSC 84054;Primostat;Depostat (TN);Gestronol caproate;Gestronol hexanoate
- CBNumber:
- CB9718151
- Molecular Formula:
- C26H38O4
- Molecular Weight:
- 414.58
- MDL Number:
- MFCD00867860
- MOL File:
- 1253-28-7.mol
| Melting point | 123-124° |
|---|---|
| alpha | D +13° (chloroform) |
| Boiling point | 453.43°C (rough estimate) |
| Density | 1.11 |
| refractive index | 1.4840 (estimate) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| form | Solid |
| color | Off-White to Pale Beige |
| FDA UNII | U38E620NS6 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H351-H360-H362 |
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501-P201-P260-P263-P264-P270-P308+P313 |
GESTONORONE CAPROATE price
GESTONORONE CAPROATE Chemical Properties,Uses,Production
Originator
Depostat,Schering AG
Uses
Gestonorone Capronate, is a pro-drug of Gestonorone, which is a steroidal progestin. It has been shown to have accelerated body weight gain and caused the atrophy of the prostate, uterus, and seminal vesicles in rats. It is also an inhibitor of the reductive pathway of Testosterone (T155000) metabolism.
Uses
Gestonorone Caproate, is a pro-drug of Gestonorone, which is a steroidal progestin. It has been shown to have accelerated body weight gain and caused the atrophy of the prostate, uterus, and seminal vesicles in rats. It is also an inhibitor of the reductive pathway of Testosterone (T155000) metabolism.
Definition
ChEBI: Gestonorone caproate is an organic molecular entity.
Manufacturing Process
2 Methods of producing of 17-α-hydroxyl-19-norprogesteron-17-capronate:
1. To a solution of 1.0 g 17-α-hydroxy-19-norprogesteron in 32 ml capronic
acid anhydride 1.32 g p-toluesulfonate (1 mole hydrate) were added, and
allowed to stand for 3 h at 37°C. To the solution 1.43 ml conc. hydrochloric
acid in 143 ml methanol were added and all this also for 1 h was left under
N2. Then mixture was washed with water and treated with ether. Ether extract
was washed with water, and dried with Na2SO4. After that ether was distilled
and residue was recrystallised with isopropyl ether. 1.1 g of 17-α-hydroxyl-19-
norprogesteron-17-capronate was obtained, melting point 123°-124°C.
2. 2.0 g 3-methoxy-17α-hydroxy-17β-acetyl-δ2,5(10)-oestradien, 60 ml
capronic acid anhydride, 2.6 g p-toluensulfonate and 18.0 g water were mixed
and left for 6 h at room temperature. Then solution obtained was treated
ether and sodium bicarbonate and washed with water. Etheral solution was
dried over sodium sulfate. After distillation of ether 3.1 g 3,17α-dihydroxy-
δ3,5-19-norpregnadien-3,17-dicapronate was produced.
To solution of 3.1 g 3,17α-dihydroxy-δ3,5-19-norpregnadien-3,17-dicapronate
in 250 ml methanol 2.5 g conc. hydrochloric acid were added and mixture was
left for 1 h. Then mixture was filtered and residue was washed. After
recrystallisation with isopropyl ether 17-α-hydroxyl-19-norprogesteron-17-
capronate was obtained, melting point 121°-123°C.
Therapeutic Function
Progestin
GESTONORONE CAPROATE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9553 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29474 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| BOC Sciences | 1-631-485-4226; 16314854226 | info@bocsci.com | United States | 14059 | 65 |
| Shanghai TaoSu Biochemical Technology Co., Ltd. | 021-33632979 | info@tsbiochem.com | China | 8073 | 58 |
| BOC Sciences | 16314854226 | info@bocsci.com | United States | 9926 | 65 |
| Hubei Yangxin Medical Technology Co., Ltd. | 15374522761 | 3003392093@qq.com | China | 7851 | 55 |
| Shenzhen Polymeri Biochemical Technology Co., Ltd. | +86-400-002-6226 13028896684 | sales@rrkchem.com | China | 55672 | 58 |
| Energy Chemical | 021-58432009 400-005-6266 | marketing1@energy-chemical.com | China | 44894 | 58 |
View Lastest Price from GESTONORONE CAPROATE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-06-03 | GESTONORONE CAPROATE USP/EP/BP
1253-28-7
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited |
-

- GESTONORONE CAPROATE USP/EP/BP
1253-28-7
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited