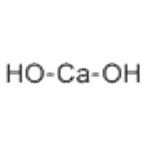
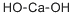Calcium hydroxide
- CAS No.
- 1305-62-0
- Chemical Name:
- Calcium hydroxide
- Synonyms
- Ca(OH)2;HYDRATED LIME;SLAKED LIME;LIME WATER;carboxide;CARBIDE LIME;CALCIUM HYDRATE;Calciumhydroxid8;Calcium Hydroxide Topical Solution;calvit
- CBNumber:
- CB9853016
- Molecular Formula:
- CaH2O2
Lewis structure
2.png)
- Molecular Weight:
- 74.09
- MDL Number:
- MFCD00010901
- MOL File:
- 1305-62-0.mol
- MSDS File:
- SDS
| Melting point | 580 °C |
|---|---|
| Boiling point | 2850 °C |
| Density | 2.24 g/mL at 25 °C (lit.) |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 1.7g/l |
| form | Solid |
| pka | 12.6[at 20 ℃] |
| color | White |
| Odor | Odorless |
| PH Range | 12.4 |
| PH | 11.27(1 mM solution);12.2(10 mM solution);12.46(100 mM solution); |
| Water Solubility | 1.65 g/L (20 ºC) |
| Sensitive | Air Sensitive |
| Merck | 14,1673 |
| Solubility Product Constant (Ksp) | pKsp: 5.26 |
| Exposure limits |
ACGIH: TWA 5 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: TWA 5 mg/m3 |
| Stability | Stable. Incompatible with strong acids. |
| CAS DataBase Reference | 1305-62-0(CAS DataBase Reference) |
| FDA 21 CFR | 184.1205; 582.1205; 310.545 |
| Substances Added to Food (formerly EAFUS) | CALCIUM HYDROXIDE |
| SCOGS (Select Committee on GRAS Substances) | Calcium hydroxide |
| EWG's Food Scores | 1-4 |
| FDA UNII | PF5DZW74VN |
| NIST Chemistry Reference | Calcium dihydroxide(1305-62-0) |
| EPA Substance Registry System | Calcium hydroxide (1305-62-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H318-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,C | |||||||||
| Risk Statements | 41-34-37/38 | |||||||||
| Safety Statements | 26-39-45-36/37/39-27 | |||||||||
| RIDADR | 3262 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | EW2800000 | |||||||||
| F | 34 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2825 90 19 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 orally in rats: 7.34 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Calcium hydroxide price More Price(57)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 239232 | Calcium hydroxide ACS reagent, ≥95.0% | 1305-62-0 | 2.5kg | $220 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.02119 | Calcium hydroxide precipitated (≤ 0.0005% Al) EMPROVE? ESSENTIAL USP,FCC,E 526 | 1305-62-0 | 0.5kg | $215 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.02110 | Calcium hydroxide EMPROVE? ESSENTIAL, Ph. Eur., BP, JP, USP, FCC, E 526 | 1305-62-0 | 1KG | $79 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.02110 | Calcium hydroxide EMPROVE? ESSENTIAL Ph Eur,BP,USP,JP,FCC,E 526 | 1305-62-0 | 25kg | $240 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.02111 | Calcium hydroxide for the preparation of DIN 19266 pH standard buffer solutions | 1305-62-0 | 25g | $307 | 2024-03-01 | Buy |
Calcium hydroxide Chemical Properties,Uses,Production
Chemical properties
Calcium hydroxide is a soft white crystalline, odorless powder with an alkaline, bitter taste.

calcium hydroxide powder
Uses
1. Calcium hydroxide Used for preparing bleaching powders, hard water softeners and the disinfecting and clarifying agents of tap water , as well as in building industry
2. Used for pharmacy. Used as rubber and oil industry additives. Used in lubricants for preventing coking, sludge deposition and anti-corrosion. Used as hard water softeners, disinfectants, antacids and so on.
3. Used as analytical reagents, carbon dioxide absorbents, as well as in organic synthesis
4. Used for making bleaching powder, hard water softener, depilatory, disinfectant, acid stop agent, astringent and various calcium salts.
5. Use for carbon dioxide examination and gas absorption. Used for leather hair removal, insecticide and water treatment.
Solubility in water (g/100ml)
Dissolved grams in 100 ml of water at different temperatures (° C):
0.189 g/0°C; 0.182g/10°C; 0.173 g/20°C; 0.16 g/30°C; 0.141g /40℃
8.6 x 10-2g/80 °C; 7.6 x 10-2g/90 °C
Identification test
Mix the sample with 3 to 4 times amount of water to form a uniform paste. Stand for clarification and the transent supernatant is alkaline for the litmus test.
Mix 1g of the sample with 20 ml of water, and add sufficient acetic acid to form solution. The calcium salt test (IT-10) is positive.
Content analysis
Accurately weigh about 1.5g of sample into a beaker and then gradually add 30ml of dilute hydrochloric acid (TS-117). After the sample is fully dissolved, transfer the mixture into a 500ml volumetric flask. Flush the beaker and transfer the lotion into the volumetric flask too. Then dilute to the mark with water and mix fully. Take 50.0ml of the above solution into an appropriate container and add 50.0ml of water. Add in turn 30ml of 0.05ml/L disodium EDTA, 15ml of sodium hydroxide solution (TS-224) and 300mg hydroxynaphthol blue indicator with a 50ml burette under stirring (preferably with a magnetic stirrer), and then continue titrating to the blue end. Per mL of 0.05 ml/L of EDTA disodium corresponds to 3.705 mg of calcium hydroxide.
Toxicity
ADI no restricting (FAO/WHO, 2001).
LD50 7300 mg/kg (mouse, oral).
Corrosive, weaker than lime.
GRAS (FDA, §182.1205, §184.1205, 2000).
The dust or suspension droplets of calcium hydroxide have a stimulating effect on the mucous membrane, which can cause sneezing and coughing. As alkali, it can also saponify the fat, absorb water from the skin, dissolve protein, as well as stimulate and corrode the organization. Moreover, the inhalation of lime dust may cause pneumonia.
The maximum allowable concentration of calcium hydroxide is 5 mg/m3. Once Inhaling dust, people can inhale water vapor, codeine and Judah auning, or paint mustard cream at the chest; when calcium hydroxide coming into the eyes, people should rinse the eyes as soon as possible with the running water and followed by 5% ammonium chloride solution or 0.01% CaNa2-EDTA solution, and then add 0.5% tetracaine solution dropwise. While working, people should wear dustproof fiber work clothes, gloves and dust-proof glasses to protect the respiratory organs, and paint grease ointment to prevent dust inhalation.
Usage limits
GB 2760-96: processing aids, GMP.
FAO/WHO (1984): butter and whey cream, 2g/kg (only used for adjust Ph value, calculate on anhydrous substance); grape juice and its concentrated juices (physical method for anticorrosion) baby food, edible caseinate, according to GMP.
In Japan, the maximum amount is 1.84% (calcium 1%).
Production method
Lime slaking method: first calcine limestone into calcium oxide, and the selected calcium oxide is slaked with water by the ratio of 1: (3~3.5). Then the generated calcium hydroxide solution is purified and separated to remove slag, and then centrifuged for dehydration. After further dried at 150~300℃, the above products is screened (120 mesh or more) to obtain the finished calcium hydroxide.
CaCO3 → CaO + CO2↑
CaO + H2O → Ca(OH) 2
Description
Calcium Hydroxide is a colorless crystal or white powder, obtained when CaO (called lime or quicklime) is mixed, or “slaked” with water. It can also be precipitated by mixing CaCl2 andNaOH.The nameof the natural,mineral formis “portlandite”. It is a relatively rare mineral, known from some volcanic, plutonic, and metamorphic rocks. It has also been known to be formed in burning coal dumps.
Chemical Properties
Calcium hydroxide occurs as a white or almost white, crystalline or granular powder. It has a bitter, alkaline taste. Calcium hydroxide readily absorbs carbon dioxide to form calcium carbonate.
Physical properties
Soft white crystalline powder; hexagonal; density 2.34 g/cm3; slightly bitter taste; loses water when heated at elevated temperatures (580°C); slightly soluble in water; Ksp 1.2x10-14; aqueous solution alkaline; soluble in glycerol and acids; insoluble in alcohol.
Uses
Calcium Hydroxide is a general food additive made by adding water to calcium oxide (lime). it has poor water solubility with a solubility of 0.185 g in 100 g of water at 0°c. the ph of a water solution at 25°c is approximately 12.4. it is used to promote dispersion of ingre- dients in sauces, creamed spinach, and a frozen pea/potato dish. it is used at 0.1% to stabilize the potassium iodide of iodized salt, and it is used as a neutralizer for soured cream prior to buttermaking. it is also termed hydrated lime, calcium hydrate, and slaked lime.
Uses
calcium hydroxide is an inorganic base used to adjust the pH of a product, it can also serve as a topical astringent and alkali in solutions or lotions. It can burn the skin and eyes.
Uses
Calcium hydroxide, a weak base, has many diverse uses. Among the most important ones are:Flocculants; Paints And Sealants; Mortar; Portland Cement Mortar; Lime Plaster
Other uses that have occurred in industry include:
An alkali used as a lye substitute (NaOH) in no-lye hair relaxers.
- A chemical depilatory agent found in Nair .
- A calcium supplement in mineral fortified baby formulas.
- A chemical reagent used to form a long-lasting fungicide.
- In “reef-aquariums” for adding bio-available calcium in solution for calcium-using animals such as algae, snails, tube worms and corals and also to increase the alkalinity of the water.
- In the tanning industry for neutralization of acid, the liming of hides and skins and the flocculation of resultant wastewater.
- In the oil industry for the manufacture of oil additives (salicatic, sulphatic, fenatic).
- In the chemical industry for manufacture of calcium stearate.
- In the food industry for processing water (for alcoholic and soft drinks).
- Used in the separation of sugar from sugar cane in the sugar industry.
- Used in the processing of Norwegian “Lutefisk”. Dried cod fish is soaked in a mixture of slaked lime and soda to produce a soft-fleshed fish fillet that is steamed or baked and served with potato “Lefse”.
- In the manufacture of brake ? pads, ebonite and pesticides.
- In dentistry, it is used as dressing in paste form used for antimicrobial effect during a dental root canal procedure. Calcium hydroxide is known to have a strong antimicrobial effect and is a boneregeneration stimulant.
- In the production of metals, lime is injected into the waste gas stream to neutralize acids, such as fluorides and chlorides prior to being released to atmosphere.
Definition
Lime water: A solution of calcium hydroxide in water. If carbon dioxide is bubbled through lime water a milky precipitate of calcium carbonate forms. Prolonged bubbling of carbon dioxide turns the solution clear again as a result of the formation of soluble calcium hydrogencarbonate (Ca(HCO3)2).
Production Methods
Calcium hydroxide is manufactured by adding water to calcium oxide, a process called slaking.
General Description
Odorless white granules. Sinks in water.
Air & Water Reactions
Water soluble. The amount of heat generated by hydrolysis may be large.
Reactivity Profile
The nitroparaffins, nitromethane, nitropropane, etc. form salts with inorganic bases such as Calcium hydroxide . The dry salts are explosive [Chem. Eng. news 30:2344 1952]. Bases are chemically similar to sodium hydroxide (NaOH) or sodium oxide (Na2O). They neutralize acids exothermically to form salts plus water. When soluble in water they give solutions having a pH greater than 7.0. Mixing these materials with water can generate troublesome amounts of heat as the base is dissolved or diluted. Bases react with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. Bases may initiate polymerization reactions in polymerizable organic compounds, especially epoxides). They may generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. Materials of this group often serve as catalysts. A strong base. Forms caustic solution in water [Merck 11th ed. 1989].
Hazard
Skin, eye, and upper respiratory tract irri- tant, avoid inhalation.
Health Hazard
Dust irritates eyes, nose and throat.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Calcium hydroxide is a strong alkali and is used as a pharmaceutical
pH adjuster/buffer and antacid in topical medicinal ointments,
creams, lotions, and suspensions, often as an aqueous solution (lime
water). It forms calcium soaps of fatty acids, which produce
water-in-oil emulsions (calamine liniment), and it is also used as a
topical astringent.
Calcium hydroxide is a common cosmetic ingredient in hairstraightening
and hair-removal products, and in shaving preparations.
1) In dentistry, it is used as a filling agent and in dental pastes
to encourage deposition of secondary dentine. Calcium hydroxide
was traditionally used as an escharotic in Vienna Paste.
Agricultural Uses
Calcium hydroxide, [Ca(OH)2], also called slaked lime
or hydrated lime, is a white solid that dissolves
sparingly in water. It is manufactured by adding water to
calcium oxide, a process that emits heat and is known as
slaking.
Calcium hydroxide is a cheap alkali, used for
neutralizing the acidity of acid soils and in the
manufacture of mortar, white wash, bleaching powder
and glass. It is an excellent absorbent for carbon dioxide
to produce insoluble calcium carbonate and has a
neutralizing value of 179%, compared to 100% of pure
calcium carbonate.
Safety Profile
Mildly toxic by ingestion. A severe eye irritant. A skin, mucous membrane, and respiratory system irritant. Mutation data reported. Causes dermatitis. Dust is considered to be a significant industrial hazard. A common air contaminant. Violent reaction with maleic anhydride, nitroethane, nitromethane, nitroparaffins, nitropropane, phosphorus. Reaction with polychloiinated phenols + potassium nitrate forms extremely toxic products. See also CALCIUM COMPOUNDS
Safety
Calcium hydroxide is used in oral and topical pharmaceutical
formulations. It is mildly toxic by ingestion. In the pure state,
calcium hydroxide is a severe skin, eye, and respiratory irritant, and
it is corrosive, causing burns. Typical exposure limits are TVL
5 mg/m3 in air.
LD50 (mouse, oral): 7.3 g/kg
LD50 (rat, oral): 7.34 g/kg
Potential Exposure
Calcium hydroxide is used in agriculture and in fertilizer manufacture; it is used in the formulation of mortar, plasters, and cements; it is used as a scrubbing and neutralizing agent in the chemical industry. For making insecticides, acaricides, and products to control arthropods
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least30 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, getmedical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering acorticosteroid spray.
storage
Calcium hydroxide should be stored in an airtight container, in a cool, dry, well-ventilated place. Calcium hydroxide powder may be sterilized by heating for 1 hour at a temperature of at least 1608℃.
Shipping
UN3262 Corrosive solid, basic, inorganic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name required
Purification Methods
Heat analytical grade calcium carbonate at 1000o during 1hour. Allow the resulting oxide to cool and add slowly to water. Heat the suspension to boiling, cool and filter through a sintered glass funnel of medium porosity (to remove soluble alkaline impurities). Dry the solid at 110o and crush it to a uniformly fine powder. [Ehrlich in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 934 1963.]
Incompatibilities
Incompatible with strong acids, maleic anhydride, phosphorus, nitroethane, nitromethane, nitroparaffins, and nitropropane. Calcium hydroxide can be corrosive to some metals.
Waste Disposal
Landfill or admixture with acid industrial wastes prior to lagooning
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (intravenous and subcutaneous injections; oral suspensions and tablets; topical emulsions and creams). Included in parenteral preparations licensed in the UK.
Calcium hydroxide Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7786 | 58 |
| Jiangsu Kolod Food Ingredients Co.,Ltd. | +86-518-85110578 +8618805133257 | sales3257@jskolod.com | China | 132 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
Related articles
- Introduction of Calcium hydroxide
- Calcium hydroxide is a material which has been used for a variety of purposes since its introduction into dentistry in the ear....
- Nov 23,2021
Related Qustion
- Q:Which base is more stronger NaOH or Ca(OH)2 ?
- A:Calcium hydroxide is more basic when the concentrations are the same.
- Mar 19,2024
View Lastest Price from Calcium hydroxide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-12-23 | Calcium hydroxide
1305-62-0
|
US $50.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-04-04 | Calcium hydroxide
1305-62-0
|
US $100.00 / T | 1T | 95% | 10t | Nanjing Sky Hope Tongyuan Biological Engineering Co., Ltd. | |
 |
2023-03-27 | Calcium Hydroxide
1305-62-0
|
US $28.00 / KG | 1KG | 0.99 | 5000KG/MONTH | XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD |
-

- Calcium hydroxide
1305-62-0
- US $50.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Calcium hydroxide
1305-62-0
- US $100.00 / T
- 95%
- Nanjing Sky Hope Tongyuan Biological Engineering Co., Ltd.
-

- Calcium Hydroxide
1305-62-0
- US $28.00 / KG
- 0.99
- XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD