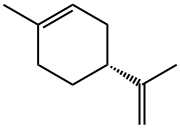
(+)-Dipentene
- CAS No.
- 5989-27-5
- Chemical Name:
- (+)-Dipentene
- Synonyms
- (R)-1-Methyl-4-(prop-1-en-2-yl)cyclohex-1-ene;D-Dipentene;citrene;imonene;d-limoneno;kautschiin;(gamma)-Carvene;(R)-1-methyl-4-(1-methylethenyl)cyclohexene;LEMOSOL;llmonene
- CBNumber:
- CB9853935
- Molecular Formula:
- C10H16
- Molecular Weight:
- 136.23
- MDL Number:
- MFCD00062991
- MOL File:
- 5989-27-5.mol
- MSDS File:
- SDS
| Melting point | -75--73°C |
|---|---|
| Boiling point | 176-177 °C(lit.) |
| alpha | 112o (10% IN ETHANOL) |
| Density | 0.842 g/mL at 20 °C(lit.) |
| vapor density | 4.7 (vs air) |
| vapor pressure | 1 mm Hg ( 20 °C) |
| refractive index |
n |
| FEMA | 2633 | D-LIMONENE |
| Flash point | 119 °F |
| storage temp. | Store below +30°C. |
| solubility | water: insoluble |
| Specific Gravity | 0.842 (20/4℃) |
| color | Colorless to Almost colorless |
| Odor | at 100.00 %. citrus orange fresh sweet |
| Odor Type | citrus |
| optical activity | [α]20/D +120°, neat |
| explosive limit | 0.7-6.1%(V) |
| Water Solubility | Miscible with alcohol,chloroform,ether and carbondisulfide.Immiscible with water. |
| Merck | 14,5493 |
| JECFA Number | 1326 |
| BRN | 2204754 |
| Stability | Stable. Flammable. Incompatible with strong oxidizing agents. |
| LogP | 4.38 at 25℃ |
| Substances Added to Food (formerly EAFUS) | D-LIMONENE |
| CAS DataBase Reference | 5989-27-5(CAS DataBase Reference) |
| FDA UNII | GFD7C86Q1W |
| IARC | 3 (Vol. 56, 73) 1999 |
| EPA Substance Registry System | D-Limonene (5989-27-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H226-H304-H315-H317-H410 | |||||||||
| Precautionary statements | P210-P273-P280-P301+P310-P303+P361+P353-P331 | |||||||||
| Hazard Codes | Xi,N,Xn | |||||||||
| Risk Statements | 10-38-43-50/53-65 | |||||||||
| Safety Statements | 24-37-60-61 | |||||||||
| RIDADR | UN 2052 3/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | GW6360000 | |||||||||
| F | 8-10-23 | |||||||||
| Autoignition Temperature | 255 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2902 19 00 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 orally in Rabbit: > 2000 mg/kg LD50 dermal Rabbit > 2000 mg/kg | |||||||||
| NFPA 704 |
|
(+)-Dipentene price More Price(35)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W263303 | (R)-(+)-Limonene ≥93% | 5989-27-5 | 1KG | $76.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | W263303 | (R)-(+)-Limonene ≥93% | 5989-27-5 | 20KG | $559 | 2024-03-01 | Buy |
| Sigma-Aldrich | CRM40422 | (R)-(+)-Limonene solution certified reference material, 2000?μg/mL in methanol, ampule of 1?mL | 5989-27-5 | 1mL | $108 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.18407 | (R)-(+)-Limonene forsynthesis | 5989-27-5 | 100mL | $36.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.18407 | (R)-(+)-Limonene forsynthesis | 5989-27-5 | 500mL | $82.5 | 2024-03-01 | Buy |
(+)-Dipentene Chemical Properties,Uses,Production
Description
d-Limonene has a pleasant, lemon-like odors free from campho raceous and turpentine-like. d-Limonene may be obtained by
steam distillation of citrus peels and pulp resulting from the pro duction of juice and cold-pressed oils, or from deterpenation of
citrus oils; it is sometimes redistilled.
The most important and widespread terpene; it is known in the el and I- optically active forms and in the optically inactive dl-form
(known as dipentene); it has been reported found in more than 300
essential oils in amounts ranging from 90 - 95% (lemon, orange,
mandarin) to as low as 1% (palmarosa);1 the most widespread form
is the d-limonene, followed by the racemic form, and then ι-
limonene.
Description
(+)-Limonene is a monoterpene that has been found in citrus oils and Cannabis and has diverse biological activities, including anticancer, antimicrobial, antioxidant, anti-inflammatory, and chemoprotective properties. It inhibits the growth of bacteria including S. aureus, B. cereus, E. faecalis, E. coli, P. aeruginosa, K. pneumoniae, M. catarrhalis, and C. neoformans with MIC values ranging from 3 to 27 mg/ml. In a human BGC-823 gastric cancer nude mouse orthotopic transplantation model, (+)-limonene inhibits tumor growth by 47.6% compared to controls and reduces the number of metastases in the liver, peritoneum, and other organs when administered by gastric perfusion at a dose of 15 ml/kg of a 1% emulsion. Dietary administration of (+)-limonene (5 and 10% in chow) reverses doxorubicin-induced decreases in glutathione (GSH) levels in rat kidney. Formulations containing (+)-limonene have been used as flavoring and fragrance agents and in the treatment of gallstones, heartburn, and gastroesophageal reflux disorder.
Chemical Properties
colourless liquid
Chemical Properties
d-, l- or dl-Limonene has a pleasant, lemon-like odor free from camphoraceous and turpentine-like notes. Limonene is the most important and widespread terpene; it is known in the d- and l- optically active forms and in the optically inactive dl-form (known as dipentene).
Occurrence
It has been reported found in more than 300 essential oils in amounts ranging from 90 to 95% (lemon, orange, mandarin) to as low as 1% (palmarosa); the most widespread form is the d-limonene, followed by the racemic form and then l-limo nene. Also reported found in ginger, nutmeg, pepper, mace, hop oil, coriander seed, calamus, dill herb, caraway seed and rosemary.
Uses
(R)-(+)-Limonene has been used to investigate the in vitro and in vivo action of limonene against Leishmania species.
Uses
Reference Standard in the analysis of herbal medicinal products
Uses
Chiral building block. Biodegradable solvent. Limonene is used as a solvent for filament-fused 3D printing, in cosmetic products, as a fragrance and as a biofuel. It is useful to promote weight loss, prevent cancer, treat cancer and treat bronchitis. It also finds use in foods, beverages, chewing gum, as a flavoring agent and a shrinking agent to dissolve polystyrene. It acts as a chiral intermediate for natural product synthesis.
Definition
ChEBI: An optically active form of limonene having (4R)-configuration.
Preparation
d-Limonene may be obtained by steam distillation of citrus peels and pulp resulting from the production of juice and cold pressed oils, or from deterpenation of citrus oils; it is sometimes redistilled.
Aroma threshold values
Detection: 4 to 229 ppb
Taste threshold values
Taste characteristics at 30 ppm: sweet, orange, citrus and terpy.
General Description
Clear colorless mobile liquid with a pleasant lemon-like odor.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
(+)-Dipentene is sensitive to exposure to light. Atmospheric oxidation can occur. (+)-Dipentene is incompatible with strong oxidizing agents. (+)-Dipentene reacts violently with (iodine pentafluoride + tetrafluoroethylene). With dry hydrogen chloride or hydrogen bromide, (+)-Dipentene forms monohalides. With aqueous hydrogen chloride or hydrogen bromide, (+)-Dipentene forms the dihalide.
Fire Hazard
(+)-Dipentene is combustible.
Agricultural Uses
Insecticide, Animal and insect repellant, Slimicide: Used as an insecticide, insect repellent, and animal repellent. Not listed for use in EU countries. Registered for use in the U.S., Canada and other countries. There are 49 global suppliers.
Trade name
BUGAWAY®[C]; BUGCHASER®[C]; CARVENE®; DOO-NOT®; HOLIDAY FIRE ANT KILLER®
Contact allergens
d-Limonene is contained in Citrus species such as citrus, orange, mandarin, and bergamot. d-limonene, used as a solvent, may be found in cleansing or in degreasing agents. Its sensitizing potential increases with prolonged air contact, which induces oxidation and leads to oxidation products. The presence of d-limonene has to be mentioned by name in cosmetics of the EU.
Biochem/physiol Actions
Limonene is a cyclie terpene from Chinese medicinal herb essential oils used in the synthesis of carvone. Limonene may be used as a shrinking agent to dissolve polystyrene. Limonene may be used in various insecticidal and insect repellant applications.
(+)-Dipentene Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Univar Solutions(China) Co., Ltd. | +8615902132654 | ivy.zhuang@univarsolutions.com | China | 654 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
View Lastest Price from (+)-Dipentene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-06 | (+)-Dipentene
5989-27-5
|
US $0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2023-08-04 | (+)-Dipentene
5989-27-5
|
US $0.00 / kg | 1kg | 99% | 20ton | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-06-15 | (+)-Dipentene
5989-27-5
|
US $13000.00 / T | 1T | 98% | 10-100mt | Baoji Guokang Bio-Technology Co., Ltd. |
-

- (+)-Dipentene
5989-27-5
- US $0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
-

- (+)-Dipentene
5989-27-5
- US $0.00 / kg
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- (+)-Dipentene
5989-27-5
- US $13000.00 / T
- 98%
- Baoji Guokang Bio-Technology Co., Ltd.