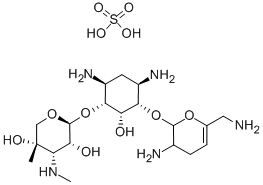
Sisomicin
- CAS No.
- 32385-11-8
- Chemical Name:
- Sisomicin
- Synonyms
- Siseptin;SISOMYCIN;Sch 13475;Sisomicin;Rickamicin;Sisomicin base;Antibiotic 6640;Sisomicin (8CI);Sisomicin USP/EP/BP;Dehydrogentamicin Cla
- CBNumber:
- CB9875243
- Molecular Formula:
- C19H37N5O7
- Molecular Weight:
- 447.53
- MDL Number:
- MFCD00865123
- MOL File:
- 32385-11-8.mol
| Melting point | 80-93°C |
|---|---|
| Boiling point | 556.56°C (rough estimate) |
| Density | 1.2777 (rough estimate) |
| refractive index | 1.7600 (estimate) |
| storage temp. | Hygroscopic, -20°C Freezer, Under Inert Atmosphere |
| solubility | Aqueous Acid (Slightly), DMSO (Slightly), Methanol (Slightly), Water (Slightly, |
| form | Solid |
| pka | 13.29±0.70(Predicted) |
| color | Pale Yellow to Yellow |
| FDA UNII | X55XSL74YQ |
| ATC code | J01GB08 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H332-H360-H312 | |||||||||
| Precautionary statements | P261-P271-P304+P340-P312-P280-P302+P352-P312-P322-P363-P501 | |||||||||
| NFPA 704 |
|
Sisomicin price More Price(19)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 21046 | Sisomicin ≥98% | 32385-11-8 | 50mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 21046 | Sisomicin ≥98% | 32385-11-8 | 100mg | $57 | 2024-03-01 | Buy |
| Cayman Chemical | 21046 | Sisomicin ≥98% | 32385-11-8 | 500mg | $230 | 2024-03-01 | Buy |
| Cayman Chemical | 21046 | Sisomicin ≥98% | 32385-11-8 | 1g | $305 | 2024-03-01 | Buy |
| Usbiological | 257387 | Sisomicin | 32385-11-8 | 100mg | $319 | 2021-12-16 | Buy |
Sisomicin Chemical Properties,Uses,Production
Description
Sisomicin was found in the culture broth of Micromonospora inyoensis by Schering-Plough Co. in 1970, following the discovery of gentamicin by the same research group. The structure and activity of sisomicin are very similar to those of gentamicin C1a, the major component of the gentamicin complex. Sisomicin shows stronger bacterial activity and lower renal and ototoxicity than gentamicin C1a .
Chemical Properties
Off-White Solid
Originator
Pathomyci n,Byk-Essex,W. Germany,1976
Uses
antibacterial, binds to ribosomes
Uses
Sisomicin is an Antibacterial. Gentamicin-like aminoglycoside antibiotic; has broad spectrum antibiotic activity.
Definition
ChEBI: Sisomycin is an amino cyclitol glycoside, an aminoglycoside antibiotic, a beta-L-arabinoside and a monosaccharide derivative.
Manufacturing Process
Tank fermentation of Micromonospora inyoensis - Germination stage 1: Under
aseptic conditions, add a lyophilized culture (or cells obtained from a slant
culture) of M. inyoensis to a 300 ml shake flask containing 100 ml of the
following sterile medium:
Beef extract 3 g
Tryptone 5 g
Yeast extract 5 g
Dextrose 1 g
Starch 24 g
Calcium carbonate 2 g
Tap water 1,000 ml
Incubate the flask and its contents for 5 days at 35°C on a rotary shaker (280
rpm, 2'' stroke).Germination stage 2: Aseptically transfer 25 ml of the fermentation medium
of Germination stage 1 to a 2-l shake flask containing 500 ml of the above
described sterile germination medium. Incubate the flask and its contents for
3 days at 28{]C on a rotary shaker (280 rpm, 2'' stroke).
Fermentation stage: Aseptically transfer 500 ml of the medium obtained from
Germination stage 2 to a 14-l fermentation tank containing 9.5 l of the
following sterile medium:
Dextrin 50 g
Dextrose 5 g
Soybean meal 35 g
Calcium carbonate 7 g
Cobalt chloride 10-6M
Tap water 1,000 ml
Antifoam (GE 60) 10 ml
Prior to sterilizing the above described medium, adjust the pH to 8.
Aerobically ferment for 66 to 90 hours while stirring at 250 rpm with air input
at 4.5 l/l/min and 25 psi. The potency of the antibiotic produced at the end of
this period reaches a peak of 150 to 225 mcm/ml and remains relatively
constant. The pH of the fermentation medium changes slightly during the
antibiotic production, varying in the range of 6.8 to 7.3.
Isolation of Antibiotic 66-40 - The whole broth is adjusted to pH 2 with 6N
sulfuric acid. (For the purpose of this example, quantities are given in terms
of 170 l of fermentation broth obtained by pooling acidified broth from 17
batches.) The acidified broth is stirred for about 15 minutes and then filtered.
Wash the mycelium with water and combine the washings with the filtrate.
Adjust the pH of the filtrate to 7 with 6N ammonium hydroxide.
To the neutralized filtrate, add sufficient oxalic acid to precipitate calcium and
filter. Reneutralize the filtrate with ammonium hydroxide. Charge the filtrate
onto a cationic exchange adsorption column containing 1,500 to 2,000 g of
IRC-50 Amberlite in its ammonium form. Discard the eluate, wash the resin
with water, and elute with 2N ammonium hydroxide. Collect 400 ml fractions
and monitor by disc testing with S. aureus ATCC-6538P. Combine active
fractions and evaporate to dryness under vacuum obtaining about 28 g of
crude Antibiotic 6640 having an activity of about 500 mcm/g.
Purification of Antibiotic 66-40 - Dissolve 28 g of crude Antibiotic 6640 in 100
ml of distilled water and charge to an anion exchange adsorption column
(Dowex 1 x 2) in the hydroxyl form. Slurry 2,000 g of the resin in water into a
column 2,5" in diameter and 36" high. Elute the column with distilled water at
a rate of about 23 ml/min collecting 100 ml fractions and monitor with a
conductivity meter and by disc testing against Staphylococcus aureus.
The disc testing provides a gross separation of antibiotic-containing eluate
fractions from those devoid of antibiotic. To insure that the fractions are
properly combined, a portion of each fraction is paper chromatographed using
the lower phase of a chloroform:methanol:17% ammonium hydroxide system
(2:1:1). Each paper is sprayed with ninhydrin and the eluates containing like
material are combined and lyophilized yielding about 5.7 g of Antibiotic 66-40
assaying about 900 mcm/mg.
brand name
Siseptin (Schering).
Therapeutic Function
Antibiotic
Antimicrobial activity
A fermentation product of Micromonospora inyoensis. A dehydro
derivative of gentamicin C1a, supplied as the sulfate salt.
It is virtually identical to gentamicin in activity and pharmacokinetic
behavior. An intramuscular dose of 1–1.5 mg/kg
achieves a peak plasma concentration of 1.5–9.0 mg/L after
0.5–1 h. It is widely distributed in body water, but concentrations
in CSF are low, even in the presence of inflammation.
The plasma half-life is 2.5 h and protein binding is <10%.
It is eliminated almost completely over 24 h in the glomerular
filtrate. Excretion decreases proportionately with renal impairment and because of the virtual identity of the behavior
of the two compounds, a gentamicin nomogram can be used
to adjust dosage. About 40% of the dose is eliminated during
a 6-h dialysis period, during which the elimination half-life
falls to about 8 h.
Mild and reversible impairment of renal function occurs in
about 5% of patients. Nephrotoxicity is more likely to be seen
in those with pre-existing renal disease or treated concurrently
with other potentially nephrotoxic drugs. Ototoxicity mainly
affecting vestibular function has been found in about 1% of
patients. Neuromuscular blockade and other effects common
to aminoglycosides including rashes, paresthesiae, eosinophilia
and abnormal liver function tests have been described.
Its uses are identical to those of gentamicin, which it closely
resembles. It is of limited availability.
General Description
This aminoglycoside antibiotic is produced by Micromonospora inyoensis. Sisomicin is very similar to gentamicin in its antimicrobial spectrum and all other properties. It is as active as gentamicin against all Enterobacteriaceae . Against Pseudomonas aeruginosa, sisomicin is more active than gentamicin, but not as active as tobramycin. There is almost complete cross-resistance between gentamicin and sisomicin with most Gram-negative bacilli. The reason for this is that sisomicin, like gentamicin, is affected by at least eight of the plasmid-coded modifying enzymes, which can be produced by Gram-negative bacilli). On the other hand, sisomicin is active against some organisms which resist gentamicin by nonenzymatic mechanisms. Sisomicin does not offer significant advantages over gentamicin. It has had limited clinical trials, and has been available commercially in Europe as a sulfate, but not in the USA, UK, and Australia. The drug dosage of sisomicin (3–6 mg/kg/day), its methods of administration, and pharmacokinetics, are similar to those of gentamicin. The toxicity of these two drugs is also probably about the same. Results of treatment of conditions, such as urinary tract infections or Gramnegative bacillary septicemias, have, in general, been similar to what would be expected from an identical gentamicin regimen.
Sisomicin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3842 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9604 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29525 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| BOC Sciences | +16314854226 | inquiry@bocsci.com | United States | 19743 | 58 |
| Hebei Duling International Trade Co. LTD | +8618032673083 | sales05@hbduling.cn | China | 15747 | 58 |
View Lastest Price from Sisomicin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-03-28 | Sisomicin
32385-11-8
|
US $30.00 / kg | 1kg | 99% | 20 tons | Hebei Duling International Trade Co. LTD | |
 |
2021-07-20 | Sisomicin
32385-11-8
|
US $1.00-1.00 / KG | 1g | 99% | 50tons | Shaanxi Dideu Medichem Co. Ltd | |
 |
2021-06-22 | Sisomicin USP/EP/BP
32385-11-8
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited |
-

- Sisomicin
32385-11-8
- US $30.00 / kg
- 99%
- Hebei Duling International Trade Co. LTD
-

- Sisomicin
32385-11-8
- US $1.00-1.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd
-

- Sisomicin USP/EP/BP
32385-11-8
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited