Sodium oxide
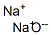
- CAS No.
- 1313-59-3
- Chemical Name:
- Sodium oxide
- Synonyms
- Na2O;Natriumoxid;Oxybissodium;SODIUM OXIDE;disodium oxide;SODIUM MONOXIDE;SodiuM oxide 80%;sodiumoxide(na2o);disodium oxygen(2-);Sodium oxide pwoder
- CBNumber:
- CB4149921
- Molecular Formula:
- Na2O
- Molecular Weight:
- 61.98
- MOL File:
- 1313-59-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/17 17:11:57
| Melting point | >400°C |
|---|---|
| Boiling point | sublimes at 1274℃ [HAW93] |
| Density | 2.27 g/mL at 25 °C (lit.) |
| vapor pressure | 0Pa at 20℃ |
| solubility | reacts with H2O |
| form | Beads |
| color | White to gray |
| Specific Gravity | 2.27 |
| Water Solubility | Soluble in water. |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,8651 |
| Stability | Stable. Reacts violently with water, acids and with many other compounds. Store under dry inert gas. May lead to fire in contact with combustible material. |
| CAS DataBase Reference | 1313-59-3(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium oxide (1313-59-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS03,GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H271-H314 | |||||||||
| Precautionary statements | P210-P220-P260-P280-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | O,C | |||||||||
| Risk Statements | 8-14-35-34 | |||||||||
| Safety Statements | 8-27-39-43-45-36/37/39-26 | |||||||||
| RIDADR | UN 3085 5.1/PG 1 | |||||||||
| WGK Germany | 1 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| NFPA 704 |
|
Sodium oxide price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 645613 | Sodium oxide 80% | 1313-59-3 | 50G | ₹8757.43 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 645613 | Sodium oxide 80% | 1313-59-3 | 250G | ₹16475.65 | 2022-06-14 | Buy |
| ottokemi | S 2210 | Sodium oxide 80% | 1313-59-3 | 50gm | ₹18099 | 2022-05-26 | Buy |
| ottokemi | S 2210 | Sodium oxide 80% | 1313-59-3 | 100gm | ₹49005 | 2022-05-26 | Buy |
Sodium oxide Chemical Properties,Uses,Production
Chemical Properties
white to grey granules or powder
Uses
Sodium oxide is used in chemical manufacturing, ceramics and glasses. Ethylbenzene dehydrogenation and carbon dioxide shift-reaction occurs using a sodium oxide/alumina catalyst. It is employed as catalyzing gasification of CO2 on carbon.
Definition
A white solid formed by burning sodium in a deficiency of oxygen or alternatively by reducing sodium peroxide or sodium hydroxide with the requisite amount of sodium. It reacts violently with water to form sodium hydroxide and with acids to form solutions of their salts. Sodium monoxide forms cubic crystals. It dissolves in liquid ammonia to give a mixture of sodamide and sodium hydroxide.
General Description
Sodium oxide is a strong alkaline oxide, commonly used as an active flux in ceramic glazes.
Sodium oxide Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Otto Chemie Pvt Ltd | +91-2222070099 +91-2266382599 | Maharashtra, India | 429 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| PK Chlorochem Private Limited | 08048372472Ext 860 | Vadodara, India | 109 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21687 | 55 | Inquiry |
| career henan chemical co | +86-0371-86658258 15093356674; | China | 29914 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 9020 | 58 | Inquiry |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | China | 33350 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 57511 | 58 | Inquiry |
1313-59-3(Sodium oxide)Related Search:
1of4
chevron_right




