アレクチニブ塩酸塩
アレクチニブ塩酸塩 物理性質
- 貯蔵温度 :
- Store at -20°C
- 溶解性:
- DMSO:3.5(Max Conc. mg/mL);6.74(Max Conc. mM)
- 外見 :
- Solid
- 色:
- White to Off-White
- 安定性::
- Hygroscopic
安全性情報
- リスクと安全性に関する声明
- 危険有害性情報のコード(GHS)
| 絵表示(GHS) |

|
| 注意喚起語 |
|
| 危険有害性情報 |
| コード |
危険有害性情報 |
危険有害性クラス |
区分 |
注意喚起語 |
シンボル |
P コード |
| H341 |
遺伝性疾患のおそれの疑い |
生殖細胞変異原性 |
2 |
警告 |
|
P201,P202, P281, P308+P313, P405,P501 |
| H361 |
生殖能または胎児への悪影響のおそれの疑い |
生殖毒性 |
2 |
警告 |
|
P201, P202, P281, P308+P313, P405,P501 |
| H373 |
長期にわたる、または反復暴露により臓器の障 害のおそれ |
特定標的臓器有害性、単回暴露 |
2 |
警告 |
|
P260, P314, P501 |
|
| 注意書き |
| P201 |
使用前に取扱説明書を入手すること。 |
| P202 |
全ての安全注意を読み理解するまで取り扱わないこ と。 |
| P260 |
粉じん/煙/ガス/ミスト/蒸気/スプレーを吸入しないこ と。 |
| P281 |
指定された個人用保護具を使用すること。 |
| P308+P313 |
暴露または暴露の懸念がある場合:医師の診断/手当てを 受けること。 |
| P314 |
気分が悪い時は、医師の診断/手当てを受けること。 |
| P405 |
施錠して保管すること。 |
| P501 |
内容物/容器を...に廃棄すること。 |
|
アレクチニブ塩酸塩 価格
| メーカー |
製品番号 |
製品説明 |
CAS番号 |
包装 |
価格 |
更新時間 |
購入 |
アレクチニブ塩酸塩 化学特性,用途語,生産方法
効能
抗悪性腫瘍薬, 未分化リンパ腫キナーゼ(ALK)阻害薬
商品名
アレセンサ (中外製薬)
説明
Alectinib hydrochloride, developed by Chugai Pharmaceutical/
Hoffman-La Roche under the trade name Alecensa®, was approved
in Japan in April 2014 for the treatment of anaplastic lymphoma
kinase (ALK) fusion-gene positive, unresectable, advanced, or
recurrent non-small cell lung cancer (NSCLC). The compound is
a highly selective second-generation ALK inhibitor, and while
alectinib currently remains a focus of further development in Europe
and the U.S., the compound has been granted orphan drug designation
in Japan after showing a 93.5% objective response rate in
phase II clinical trials. In addition to providing rapid treatment
response time in a majority of patients, trials showed a 76%
2-year progression-free survival rate. Since the initial approval
of crizotinib—the first ALK inhibitor indicated for treatment of ALKrearranged
NSCLC —patients treated with crizotinib have shown
remarkable improvement as compared to treatment with other
chemotherapeutic methods,21 although drug resistance has shown
to be a major side effect of this therapy. Preliminary preclinical
and clinical studies of alectinib have shown significant promise
for overcoming drug resistance developed with other ALK
inhibitors.
使用
CH5424802 Hydrochloride is a highly selective and potent anaplastic lymphoma kinase (ALK) inhibitor capable of blocking the resistant gatekeeper mutant, which results in reduced cell growth. Also is an intermediate of Alectinib (C183360), a highly selective and potent anaplastic lymphoma kinase (ALK) inhibitor capable of blocking the resistant gatekeeper mutant, which results in reduced cell growth.
合成
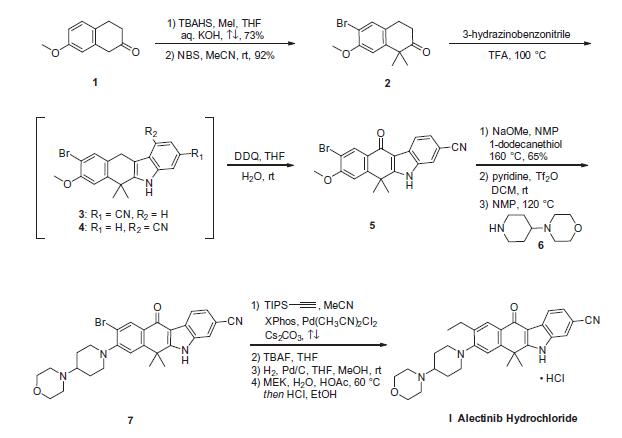
The synthetic route to alectinib as reported by Chugai
begins with 7-methoxy-2-tetralone (1). Bis-methylation
with tetrabutylammonium hydrogen sulfide (TBAHS)/aq KOH/MeI
followed by bromination with N-bromosuccinimide (NBS) provided
the bromo-tetralone 2 in 67% yield over the two steps. Further
reaction of 2 with 3-hydrazinobenzonitrile/trifluoroacetic acid (TFA) led to formation of the desired Fischer indole product,
albeit as a 1:1 mixture of regioisomers (3/4), which were carried
forward as a mixture to oxidation with 2,3-dichloro-5,6-dicyano-
1,4-benzoquinone (DDQ). It is important to note that although representative
procedures are published describing the conversion of
2 to alectinib (I), no yields were provided for these transformations.
Following oxidation, the desired product 5 could be isolated
as a single isomer via precipitation from the crude reaction mixture.
Installation of the 4-morpholino-piperidine moiety took place
in three transformations from 5, beginning with 1-dodecanethiol/
N-methyl-2-pyrrolidone (NMP)/NaOMe-facilitated methyl cleavage.
The corresponding phenol was then readily converted to the
triflate intermediate and displaced with 4-(piperidin-4-yl)morpholine
(6) at elevated temperature, providing intermediate 7. Crosscoupling
of the bromide 7 with ethynyl triisopropylsilane under
Pd-catalyzed cross-coupling conditions (Pd(CH3CN)2Cl2/2-dicyclohexylphosphino-
20,40,60-triisopropylbiphenyl (XPhos), reflux) followed
by cleavage of the resulting alkylsilane with
tetrabutylammonium fluoride (TBAF) yielded the ethynyl precursor
to alectinib. Hydrogenation of this unsaturated system under
standard conditions (H2, Pd/C) followed by HCl salt formation furnished
the final drug target alectinib hydrochloride (I).
IC 50
1.9 nM
アレクチニブ塩酸塩 上流と下流の製品情報
原材料
準備製品
アレクチニブ塩酸塩 生産企業
Global( 164)Suppliers
1256589-74-8(アレクチニブ塩酸塩)キーワード:
- 1256589-74-8
- 9-ethyl-6,6-diMethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-5a,6,11,11a-tetrahydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride
- CH-5428402
- 9-Ethyl-6,11-dihydro-6,6-dimethyl-8-[4-(4-morpholinyl)-1-piperidinyl]-11-oxo-5H-benzo[b]carbazole-3-carbonitrile hydrochloride (1:1)
- AF-802 Hydrochloride
- CH5424802 Hydrochloride
- CH-5424802 Hydrochloride
- RG-7853 Hydrochloride
- RO-5424802 Hydrochloride
- CH 5424802,Alectinib(HCl)
- CH-5428402 HCl
- CH5424802 HCl (AF 802 HCl, Alectinib HCl)
- CH5424802 HCl salt, Alectinib HCl salt, AF802 HCl salt
- Alectinib HCl salt
- 9-ethyl-6,6-dimethyl-8-(4-morpholin-4-ylpiperidin-1-yl)-11-oxo-5H-benzo[b]carbazole-3-carbonitrile,hydrochloride
- Alectinib (CH5424802) HCl
- Alectinib (CH5424802) hydrochloride
- 9-ethyl-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile monohydrochloride monohydrate
- Alectinib Hydrochloride (Alecensa)
- 5H-Benzo[b]carbazole-3-carbonitrile, 9-ethyl-6,11-dihydro-6,6-dimethyl-8-[4-(4-morpholinyl)-1-piperidinyl]-11-oxo-, hydrochloride (1:1)
- CH5424802 HCl
- 9-ethyl-6,6-dimethyl-8-[4-(morpholin-4-yl)piperidin-1-yl]-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride
- Alectinib HCl (ALECENSA, AF-802, CH-5424802, RO-5424802)
- 9-Ethyl-6,6-dimethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-5a,6,11,11A-tetrahydro-5H-benzo[b]carbazole-3-carbonitrile HCL
- Alectinib HCl
- Alectinib Hydrochloride
- CPD0098(HCl)
- 9-Ethyl-6,11-dihydro-6,6-dimethyl-8-4-morpholin-4-yl-piperidin-1-yl-11-oxo-5H-benzobcarbazol-3-carbonitrile HCl
- Alectinib hydrochloride (JAN)
- アレクチニブ塩酸塩
- アレクチニブ塩酸塩 (JAN)