酢酸ナトリウム 化学特性,用途語,生産方法
外観
白色、結晶~結晶性粉末
種類
酢酸ナトリウムには、医薬品試験用・食品添加物・試薬特級・1級・工業用などの種類があります。販売されている容量は25グラム、100グラム、500グラム、20キログラムなどです。酢酸ナトリウム溶液も100ミリリットルや500ミリリットルの容量で販売されています。
定義
本品は、酢酸(*)のナトリウム塩であり、次の化学式で表される。
参照表示名称:酢酸
性質
酢酸ナトリウムは弱酸 (酢酸)と強塩基 (水酸化ナトリウム) の塩です。水溶液中では酢酸イオン (CH3COO-) とナトリウムイオン(Na+) に電離しています。
CH3COONa → CH3COO- + Na+
酢酸イオンは、そのままの状態で存在していることはあまりありません。水溶液中では、水の電離により生じた水素イオン (H+) と結びつき、酢酸分子になりやすい性質があります。
H2O ⇄ H+ + OH-
CH3COO- + H+ ⇄ CH3COOH
H+が消費されて、水溶液中の水酸化物イオン (OH-) がH+よりも多くなるため、酢酸ナトリウムの水溶液は弱塩基性を示します (pH7~9) 。酢酸ナトリウムは水によく溶けます。20℃の水100グラムに対する酢酸ナトリウムの溶解度は46.4です。潮解性を有するため、乾燥した場所での保管が必要です。
溶解性
水に溶けやすく、エタノールにやや溶けやすい。
解説
酢酸ナトリウム,CH3COONa(82.03).酢酸と水酸化ナトリウムの中和,または酢酸カルシウムと硫酸ナトリウムの複分解により三水和物が得られる.三水和物は無色の単斜晶系結晶.風解性で,融点59 ℃.120~250 ℃ で乾燥すると無水物となる.無水物は白色の単斜晶系結晶.密度1.528 g cm-3.融点320 ℃.吸湿性で,水に易溶,エタノールに微溶.分析試薬,緩衝剤,食品防腐剤,医薬品,写真,めっき工業,媒染剤,脱水剤,有機合成などに用いられる.
用途
核酸のエタノール沈殿、緩衝液調製用等。
用途
染料、医薬品の製造研究用、脱水剤。
用途
酢酸ナトリウム(無水)は透析薬原料?食品添加物などに使用されています。
化粧品の成分用途
緩衝剤、香料
合成
酢酸ナトリウムは、以下の方法で合成できます。
- 酢酸との反応
CH3COOH + NaOH → CH3COONa + H2O
- 酢酸と炭酸ナトリウムの反応
CH3COOH + Na2CO3 → CH3COONa + NaHCO3
- 酢酸カルシウムと硫酸ナトリウムの反応
Ca(CH3COO)2 + Na2SO4 → 2CH3COONa + CaSO
使用上の注意
吸湿性あり
説明
Sodium acetate (CH3COONa) is the sodium salt of acetic acid. It appears as a colorless deliquescent salt with a wide range of applications. In industry, it can be used in textile industry to neutralize sulfuric acid waste streams and as a photoresist upon using aniline dyes. In concrete industry, it can be used as a concrete sealant to mitigate the water damage. In food, it can be used as a seasoning. It can also be used as a buffer solution in lab. In addition, it is also used in heating pads, hand warmers and hot ice. For laboratory use, it can be produced by the reaction between acetate with the sodium carbonate, sodium bicarbonate and sodium hydroxide. In industry, it is prepared from the glacial acetic acid and sodium hydroxide.
化学的特性
Sodium acetate, CH3COONa, also abbreviated NaOAc , also sodium ethanoate, is the sodium salt of acetic acid, was made by the reaction of acetic acid with sodium carbonate. It is soluble in water but less so in alcohol. This colourless salt has a wide range of uses. Sodium acetate was used as a pH modifier for toning baths.
物理的性質
Anhydrous salt is a colorless crystalline solid; density 1.528 g/cm
3; melts at 324°C; very soluble in water; moderately soluble in ethanol. The colorless crystalline trihydrate has a density 1.45 g/cm
3; decomposes at 58°C; is very soluble in water; pH of 0.1M aqueous solution is 8.9; moderately soluble in ethanol, 5.3 g/100mL.
天然物の起源
Acetic acid or acetates are present in most plant and animal tissues in small, but detectable amounts
使用
Used as buffers.
Acidity regulation (buffering)
Sodium acetate mixed with acetic acid forms a pH buffer, which can be used to stabilise the pH of foods in the pH-range from 3 to 6. The table below gives indicative values of the composition needed to give a certain pH. The mixtures below can be diluted at least 10 times with minimum effect on pH, however, the stability decreases.
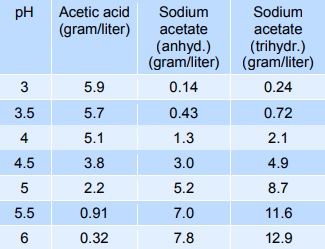
製造方法
Sodium acetate is prepared by reacting sodium hydroxide or sodium carbonate with acetic acid in aqueous solution. The solution is evaporated to obtain hydrated crystals of sodium acetate.
NaOH + CH3COOH → CH3COONa + H2O
Na2CO3 + CH3COOH → 2CH3COONa + CO2 + H2O
合成
For laboratory use, sodium acetate is very inexpensive, and is usually purchased instead of being synthesized. It is sometimes produced in a laboratory experiment by the reaction of acetic acid (ethanoic acid) with sodium carbonate, sodium bicarbonate, or sodium hydroxide. These reactions produce aqueous sodium acetate and water. Carbon dioxide is produced in the reaction with sodium carbonate and bicarbonate, and it leaves the reaction vessel as a gas (unless the reaction vessel is pressurized). This is the well-known "volcano" reaction between baking soda (sodium bicarbonate) and vinegar.
CH
3COOH + NaHCO
3 → CH
3COONa + H
2O + CO
2 Industrially, sodium acetate is prepared from glacial acetic acid and sodium hydroxide.
CH
3COOH + NaOH → CH
3COONa + H
2O.
定義
ChEBI: Sodium acetate is an organic sodium salt. It contains an acetate.
反応性
Sodium acetate can be used to form an ester with an alkyl halide such as bromo ethane:
CH
3COONa + Br CH
2CH
3→ CH
3COOCH
2CH
3+ NaBr
Caesium salts catalyze this reaction.
一般的な説明
Sodium Acetate is reported to inhibit the growth of
Listeria monocytogenes.
反応プロフィール
When sodium acetate reacts with strong acids, irritating, noxious vapors of acetic acid are usually produced. Sodium acetate is sufficiently basic to catalyze the violent polymerization of diketene, perhaps as well as other reactive dimers that are susceptible to polymerization in the presence of a mild base.
使用用途
酢酸ナトリウムは主に下記の用途で使用されます。
1. 試薬
酢酸ナトリウムは試薬として用いられ、緩衝液 (酸や塩基を少量加えてもpHをほぼ一定に保つ働きがある溶液) がその一例です。酢酸と酢酸ナトリウムを混合して作られる緩衝液は、HPLC (高速液体クロマトグラフ) 分析などに用いられています。
2. 食品添加物
酢酸ナトリウムは食品添加物です。微生物の生育を抑制する作用があることから、食品の保存期間を延ばす目的で使用されます。また、酸味を調整する物質としてソース類やマヨネーズにも使われています。
3. 媒染剤
酢酸ナトリウムは、布への染料の付着を促進する働きがある物質です。
その他、医薬品、写真用薬品、メッキ薬剤、脱水剤、有機合成触媒など幅広い用途で使用されています。
生物活性
Commonly used laboratory reagent
安全性プロファイル
Poison by intravenous route. Moderately toxic by ingestion. A skin and eye irritant. Migrates to food from packagmg materials. Violent reaction with F2, m03, diketene. When heated to decomposition it emits toxic fumes of Na2O.
純化方法
Crystallise it from acetic acid and keep it under vacuum for 10hours at 120o. Alternatively, it is crystallised from aqueous EtOH, as the trihydrate. This material can be converted to anhydrous salt by heating slowly in a porcelain, nickel or iron dish, so that the salt liquefies. Steam is evolved and the mass again solidifies. Heating is now increased so that the salt melts again. (NB: if it is heated too strongly, the salt can char; avoid this.) After several minutes, the salt is allowed to solidify and is cooled to a convenient temperature (in a desiccator) before being powdered and bottled. The water content should now be less than 0.02%. [Beilstein 2 II 113, 2 III 184, 2 IV 109.]
酢酸ナトリウム 上流と下流の製品情報
原材料
準備製品
イブジラスト
7H-ジベンゾ[c,g]カルバゾール
4-METHYL-2-METHYLSULFANYL-PYRIMIDINE-5-CARBOXYLIC ACID ETHYL ESTER
デヒドロコール酸
8-ブロモ-3,7-ジメチル-3,7-ジヒドロ-1H-プリン-2,6-ジオン
2-(メチルチオ)-4-(トリフルオロメチル)ピリミジン-5-カルボン酸エチル
2-(メチルチオ)エタノール
3-ピリダジンカルボン酸
酢酸 シトロネリル
酢酸テルピニル
3-(3-ニトロフェニル)プロピオン酸
(1β,5β)-8-(1-メチルエチル)-8-アザビシクロ[3.2.1]オクタン-3α-オール
2-ニトロフラン-5-カルボニトリル
6-アザチミン
rel-2α*-イソプロピル-5β*-メチルシクロヘキサン-1β*-オールアセタート
2酢酸·ナトリウム
2-ニトロジフェニルアミン
4-アミノテトラヒドロピラン塩酸塩
酢酸シンナミル
3-NITRO-PYRIDINE-2-CARBOXYLIC ACID
3-アセチル-4-ヒドロキシ-2H-1-ベンゾピラン-2-オン
1,2-エポキシシクロオクタン
トリプシンインヒビター, 大豆由来
レボシメンダン
アシッド ブルー 92
(2S,5R)-3,3-ジメチル-6α-[[(5-メチル-3-フェニルイソオキサゾール-4-イル)カルボニル]アミノ]-7-オキソ-4-チア-1-アザビシクロ[3.2.0]ヘプタン-2β-カルボン酸ナトリウム
酢酸 4-メトキシベンジル
エチニルフェロセン
酢酸(-)-メンチル
3-アセトキシベンゾ[B]フラン
5-アセトキシメチルフルフラール
N-(1,3-ジチオラン-2-イリデン)アミドりん酸ジエチル
C.I.バットブラック9
アセトメナフトン
Synthetic greasing agent
メチル trans-2-ヘキサノエ-ト
メチル エロー
3-(4-フルオロフェニル)ピラゾール-4-カルボキシアルデヒド
1H-ベンゾトリアゾール-1-イルオキシトリス(ジメチルアミノ)ホスホニウムヘキサフルオロホスファート
トリス(ジベンジリデンアセトン)ジパラジウム(0)