2-ヒドロキシ安息香酸 化学特性,用途語,生産方法
外観
白色, 結晶〜結晶性粉末
定義
本品は、次の化学式で表される芳香族カルボン酸である。
性質
サリチル酸は常温常圧で固体で、昇華性のある無色の針状結晶です。融点は159℃で、2.6kPaでの沸点は約211℃であり、引火点と発火点はそれぞれ157°Cと545°Cです。
、エーテルに溶けやすく、水に溶けにくいです。無臭でわずかな酸味があります。
溶解性
水に微溶 (1.8g/100ml水, 20℃), 熱湯に可溶。エタノール, エーテルに易溶。エタノール及びアセトンに溶けやすく、水に溶けにくい。
解説
o-hydroxybenzoic acid.C7H6O3(138.12).種々の植物中にこのまま,または誘導体の形で存在し,とくに冬緑油中にはメチルエステルとして含まれている.サリチル酸は,工業的には,フェノールと二酸化炭素からのコルベ-シュミット反応により合成される.無色の結晶.融点159 ℃,沸点約211 ℃(2.6 kPa).減圧下に昇華し,また水蒸気蒸留される.エタノール,エーテル,アセトンに易溶,水,ベンゼンに可溶.Ka 1.06×10-3(25 ℃).塩化鉄(Ⅲ)で紫色を呈する.清酒,果実酒,酢などの食品の防腐剤として用いられ,また染料,とくに媒染アゾ染料の中間物としても用途がある.また,サリチル酸の誘導体はアスピリンをはじめとして医薬品に供されるものが多い.LD50 1.3 g/kg(ウサギ,経口).森北出版「化学辞典(第2版)
用途
角質剥離、抗かび剤
用途
オルトヒドロキシ安息香酸の別名。無色の結晶。融点159℃,沸点211℃(20mmHg)。水に可溶,エチルアルコール,エーテルに易溶。塩化第二鉄塩水溶液で紫色を呈する。フェノールのナトリウム塩に二酸化炭素を加圧下で反応させてつくる。防腐剤,染料中間体,医薬原料などとして重要。たとえば,アセチル化すればアセチルサリチル酸(商品名アスピリン)。清酒,酢などの食品防腐剤として使用されていたが,現在は食品添加物としての使用は禁止。
殺菌剤、角質軟化剤として用いる。解熱鎮痛消炎作用をもつが、内服や注射にはサリチル酸ナトリウムが用いられ、サリチル酸そのものは消化器障害が著しいため内服には用いられず、外用のみである。うおのめ、いぼとりに用いられるサリチル酸絆創膏(ばんそうこう)には50%含有され、白癬(はくせん)(水虫)や乾癬など皮膚糸状菌症には2~10%の軟膏や塗布液が用いられる。また脱毛症や腋臭(えきしゅう)症(わきが)、汗疹(かんしん)(あせも)などの治療剤にも配合されている。
構造
サリチル酸は、ベンゼンの持つ1個の水素原子がカルボキシ基に置換され、カルボキシ基のオルト位にある1個の水素原子が水酸基に置換された構造をしています。
化学式はC7H6O3で、モル質量は138.12g/molです。示性式はHOC6H4COOHで、密度は1.443g/cm3です。
合成
サリチル酸の合成法
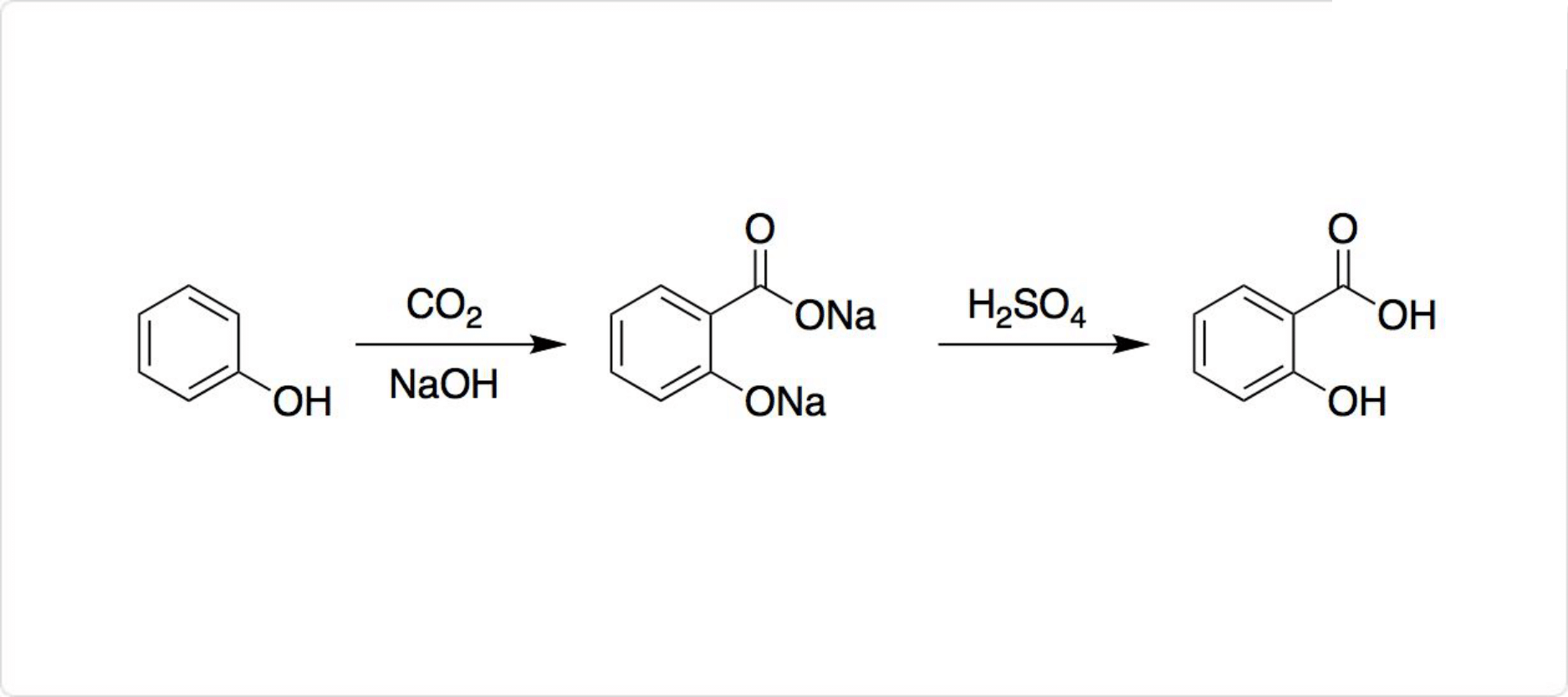
図2. サリチル酸の合成
サリチル酸は比較的簡単な化学構造を有するので、ヤナギから抽出する必要はなく、全合成が容易です。工業的には、高温高圧下でナトリウムフェノキシドにを反応させた後、さらにやを加えることでサリチル酸を遊離させています。
この一連の反応を利用したサリチル酸の製法は、コルベシュミット法 (英: Kolbe-Schmitt reaction) と呼ばれています。それに対して、同条件でカリウムフェノキシドと二酸化炭素を反応させると、カルボキシ基をパラ位に導入可能です。
パラヒドロキシ安息香酸が、およそ90%生成します。メチルエステルからブチルエステルまではパラベンと呼ばれており、防腐剤として利用されています。
化粧品の成分用途
パーマネント.ウェーブ用還元剤、ヘアコンディショニング剤、変性剤、剥離剤、角質柔軟剤、抗アクネ剤、抗フケ剤、皮膚コンディショニング剤、香料
効能
抗真菌薬, 角質溶解薬
商品名
サリチル酸 (健栄製薬); スピール膏 (ニチバン)
説明
Salicylic acid (from Latin salix, willow tree, from the bark of which the substance used to be obtained) is a mono hydroxy benzoic acid, a type of phenolic acid and a beta hydroxy acid. This colorless crystalline organic acid is widely used in organic synthesis and functions as a plant hormone. It is derived from the metabolism of salicin. In addition to being an important active metabolite of aspirin (acetylsalicylic acid), which acts in part as a prod rug to salicylic acid, it is probably best known for its use in anti-acne treatments. The salts and esters of salicylic acid are known as salicylates.
化学的特性
Salicylic acid has the formula C
6H
4(OH) COOH, where the OH group is ortho to the carboxyl group. It is also known as 2- hydroxybenzoic acid. It is poorly soluble in water (2 g / L at 20 °C). Aspirin (acetyl salicylic acid or ASA) can be prepared by the esterification of the phenolic hydroxyl group of salicylic acid with the acetyl group from acetic anhydride or acetyl chloride.
物理的性質
Salicylic acid. Appearance: white crystalline powder. Solubility: Absolutely soluble
in ethanol, soluble in ether and chloroform, slightly soluble in water and anhydrous
ether. Stability: Stable at room temperature, discomposes into phenol and carbon
dioxide after rapidly heated. It’s partially acidic.
Aspirin. Appearance: white crystal and decomposes at 136–140? °C. Melting
point: 136?°C.?Aspirin is the acetyl derivative of salicylic acid with weak acidity. Its
acidity coefficient is 3.5 at 25?°C. Stability: Aspirin decomposes rapidly in ammonium acetate, alkali metal of acetate, carbonate, citrate or hydroxide solutions.
There are two crystal forms of aspirin including crystal form I and II.
天然物の起源
Unripe fruits and vegetables are natural sources of salicylic acid, particularly blackberries, blueberries, cantaloupes, dates, raisins, kiwi fruits, guavas, apricots, green pepper, olives, tomatoes, radish and chicory; also mushrooms. Some herbs and spices contain quite high amounts, although meat, poultry, fish, eggs and dairy products all have little to no salicylates. Of the legumes, seeds, nuts, and cereals, only almonds, water chestnuts and peanuts have significant amounts.
来歴
The Greek physician Hippocrates wrote in the 5th century BC about a bitter powder extracted from willow bark that could ease aches and pains and reduce fevers . This remedy was also mentioned in texts from ancient Sumer , Lebanon , and As syria .
The active extract of the bark, called salicin, after the Latin name for the white willow (Salix alba), was isolated and named by the German chemist Johann Andreas Buchner in 1826. Raffaele Piria, an Italian chemist was able to convert the substance into a sugar and a second component, which on oxidation becomes salicylic acid.
Salicylic acid was also isolated from the herb meadowsweet (Filipendula ulmaria, formerly classified as Spiraea ulmaria) by German researchers in 1839. While their extract was somewhat effective, it also caused digestive problems such as gastric irritation, bleeding, diarrhea, and even death when consumed in high doses.
使用
Salicylic Acid is an Impurity of Acetylsalicylic Acid (A187780). Acetylsalicylic acid Impurity B.
調製方法
Salicylic acid is biosynthesized from the amino acid phenylalanine. In Arabidopsis thaliana it can also be synthesized via a phenylalanine - independent pathway.
Sodium salicylate is commercially prepared by treating sodium phenolate ( the sodium salt of phenol ) with carbon dioxide at high pressure (100 atm ) and high temperature (390K) -a method known as the Kolbe-Schmitt reaction. Acidification of the product with sulfuric acid gives salicylic acid :
It can also be prepared by the hydrolysis of aspirin (acetylsalicylic acid) or methyl salicylate (oil of winter green) with a strong acid or base.
定義
ChEBI: A monohydroxybenzoic acid that is benzoic acid with a hydroxy group at the ortho position. It is obtained from the bark of the white willow and wintergreen leaves.
適応症
Salicylic acid is a β-hydroxy acid that penetrates into the sebaceous gland
and has comedolytic and anti-inflammatory properties. It can be used as an
adjunctive therapy and is found in cleansers, toners, masks, and peels.
Salicylic acid is keratolytic and at concentrations between 3% and 6% causes softening
of the horny layers and shedding of scales. It produces this desquamation
by solubilizing the intercellular cement and enhances the shedding of corneocytes
by decreasing cell-to-cell cohesion. In concentrations >6%, it can be destructive to
tissue. Application of large amounts of the higher concentration of salicylic acid can
also result in systemic toxicity. Salicylic acid is used in the treatment of superficial
fungal infections, acne, psoriasis, seborrheic dermatitis, warts, and other scaling dermatoses.
When it is combined with sulfur, some believe that a synergistic keratolytic
effect is produced. Common preparations include a 3% and 6% ointment with
equal concentration of sulfur; 6% propylene glycol solution (Keralyt); 5% to 20%
with equal parts of lactic acid in flexible collodion for warts (Duofilm, Occlusal);
in a cream base at any concentration for keratolytic effects; as a 60% ointment for
plantar warts; and in a 40% plaster on velvet cloth for the treatment of calluses and
warts (40% salicylic acid plaster).
製造方法
Prepared by heating sodium phenolate with carbon dioxide under pressure
一般的な説明
Odorless white to light tan solid. Sinks and mixes slowly with water.
空気と水の反応
Sublimes and forms vapor or dust that may explode [USCG, 1999].
危険性
Respiratory alkalosis, hyperkalemia,
hyperthermia, dehydration, convulsions, shock, res-
piratory paralysis, respiratory acidosis, lesions and
death from respiratory collapse; fetotoxic.
健康ハザード
Inhalation of dust irritates nose and throat. Vomiting may occur spontaneously if large amounts are swallowed. Contact with eyes causes irritation, marked pain, and corneal injury which should heal. Prolonged or repeated skin contact may cause marked irritation or even a mild burn.
使用用途
サリチル酸には、殺菌作用、消炎鎮痛作用、皮膚の角質軟化作用があります。そのため、医薬の分野で主に皮膚疾患の外用薬として利用される場合が多いです。また、サリチル酸の誘導体であるアセチルサリチル酸は、サリチル酸の鎮痛効果を保ちつつ、内服時の副作用が軽減されているので、鎮痛内服薬として広く使用可能です。
さらに、角質除去を目的として洗顔料などにも配合されているほか、染料中間物や防腐剤としても利用されています。かつては食品にも防腐剤として使われていましたが、現在は食品添加物としての使用はできません。
関連する反応
サリチル酸の反応
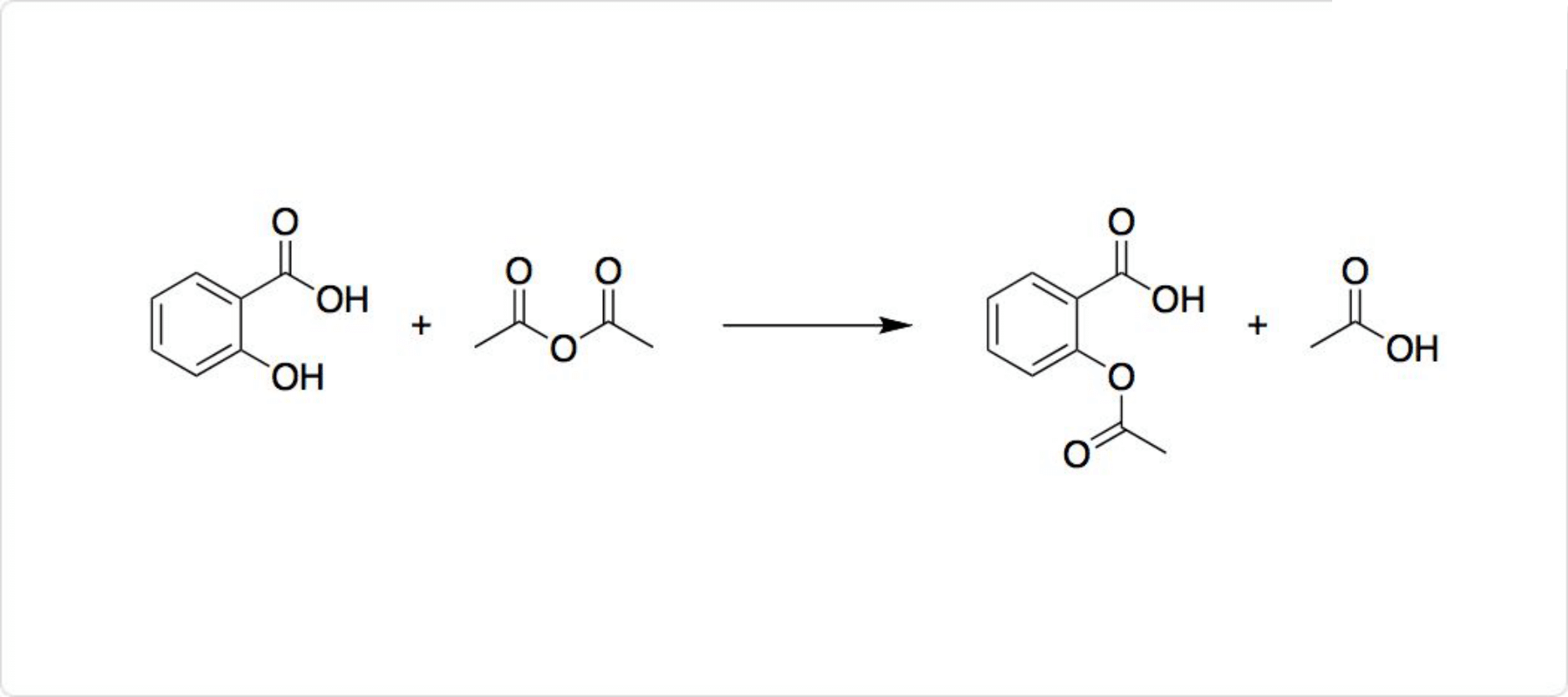
図3. サリチル酸の反応
性の水酸基を有するサリチル酸は、塩化第二鉄の水溶液により青~赤紫色の呈色反応を起こします。との反応によってアセチル化して、アセチルサリチル酸が生成します。アセチルサリチル酸は代表的な解熱鎮痛剤の1つです。
非ステロイド性抗炎症薬の代名詞と言われる医薬品であり、消炎、解熱、鎮痛、抗血小板作用などを有しています。
代謝
サリチル酸の代謝
ヒトに投与されたサリチル酸は、代謝されずにそのまま腎臓から尿中に排泄される場合もあります。例えば、大量のアセチルサリチル酸を服用した中毒時など、ヒトの血中にサリチル酸が多く含まれている状態になっても、尿中に大量のサリチル酸を排泄可能です。
特に尿のpHがアルカリ側に傾いた場合には、尿中のサリチル酸の排泄量が増加します。尿中に50μg/ml以上のサリチル酸が存在する場合には、塩化第二鉄水溶液によって呈色反応が起きます。
作用機序
Salicylic acid has been shown to work through several different pathways. It produces its anti - inflammatory effects via suppressing the activity of cyclo oxygenase (COX), an enzyme which is responsible for the production of pro - inflammatory mediators such as the prostaglandins. Notably, it does this not by direct inhibition of COX, unlike most other non-steroidal anti-inflammatory drugs (NSAIDs), but instead by suppression of the expression of the enzyme (via a yet-un elucidated mechanism) . Salicylic acid has also been shown to activate adenosine monophosphate-activated protein kinase (AMPK), and it is thought that this action may play a role in the anticancer effects of the compound and its prod rugs aspirin and salsalate. In addition, the anti diabetic effects of salicylic acid are likely mediated by AMPK activation primarily through allosteric conformational change that increases levels of phosphorylation. Salicylic acid also uncouples oxidative phosphorylation which leads to increased ADP:ATP and AMP:ATP ratios in the cell. Consequently, salicylic acid may alter AMPK activity and subsequently exert its anti-diabetic properties through altered energy status of the cell. Even in AMPK knock - out mice, however, there is an anti-diabetic effect demonstrating that there is at least one additional, yet - unidentified action of the compound.
薬理学
Aspirin is a nonsteroidal anti-inflammatory drug (NSAID). The main pharmacological effect is to inhibit prostaglandin metabolism and thromboxane synthesis by
inhibiting prostaglandin metabolism-required cyclooxygenase, via irreversible acetylation of 530 serine residues in the hydroxyl of COX-1 polypeptide chain,
which results in COX-1 inactivation, blocks the conversion of arachidonic acid into
thromboxane A2 pathway and then inhibits the platelet aggregation.
Prostaglandin is a hormone produced locally in the body. It can pass the pain to
the brain, regulate body temperature in the hypothalamus and cause inflammation.
Inhibition of prostaglandin synthesis can have antipyretic, analgesic, antiinflammatory and antirheumatic effects. The adverse effects of aspirin are mainly
gastrointestinal symptoms such as nausea, vomiting, upper abdominal discomfort or
pain. It can also cause allergic reactions, cardiotoxicity, liver and kidney damage
and Wright’s syndrome. In addition, high doses of aspirin can cause salicylic acid
reactions such as headache, dizziness, tinnitus, hearing loss and other central nervous system symptoms.
臨床応用
The clinical application of aspirin varies with the therapeutic dose. Low-dose aspirin (75–300?mg/day) has antiplatelet aggregation effect and can be used to prevent
and treat the coronary and cerebrovascular thrombosis and other postoperative
thrombosis. The middle dose of aspirin (0.5–3? g/day) has antipyretic analgesic
effects, so it is commonly used in the treatment of fever, headache, toothache, neuralgia, muscle pain and menstrual pain. High doses of aspirin (more than 4?g/day)
have anti-inflammatory and antirheumatic effects for the treatment of acute rheumatic fever and rheumatoid arthritis. In addition, aspirin is used for the treatment of
skin and mucous membrane lymphadenopathy (Kawasaki disease) in paediatric.
副作用
Salicylic acid's side
effects include erythema and scaling.
安全性プロファイル
Poison by ingestion, intravenous, and intraperitoneal routes. Moderately toxic by subcutaneous route. An experimental teratogen. Human systemic effects by skin contact: ear tinnitus. Mutation data reported. A skin and severe eye irritant. Experimental reproductive effects. Incompatible with iron salts, spirit nitrous ether, lead acetate, iodine. Used in the manufacture of aspirin. When heated to decomposition it emits acrid smoke and irritating fumes.
職業ばく露
Used as a topical keratolytic agent; in
manufacture of aspirin, salicylates, resins, as a dyestuff
intermediate; prevulcanization inhibitor; analytical reagent;
fungicide, antiseptic, and food preservative.
純化方法
It has been purified by steam distillation, by recrystallisation from H2O (solubility is 0.22% at room temperature and 6.7% at 100o), absolute MeOH, or cyclohexane and by sublimation in a vacuum at 76o. The acid chloride (needles) has m 19-19.5o, b 92o/15mm, the amide has m 133o (yellow needles from H2O), the O-acetyl derivative has m 135o (rapid heating and the liquid resolidifies at 118o), and the O-benzoyl derivative has m 132o (aqueous EtOH). [IR: Hales et al. J Chem Soc 3145 1954, Bergmann et al. J Chem Soc 2351 1950]. [Beilstein 10 IV 125.]
不和合性
iron salts; lead acetate; iodine. Forms an
explosive mixture in air.
2-ヒドロキシ安息香酸 上流と下流の製品情報
原材料
準備製品