アクロレイン (モノマー) 化学特性,用途語,生産方法
種類
アクロレインは、不安定な化合物のため、あまり製造販売されていません。ただし、研究用試薬としては若干の流通があります。
研究用試薬としては、以下のような形状で市販されています。
- 100ug/mLまたは10mg/mL水溶液
- 100ug/mLもしくは1,000ug/mLの:水=9:1溶液
- 5.0mg/mLパラジオキサン溶液
非常に不安定な化合物のため、通常、冷蔵または冷凍試薬です。重合体や酸化体が生成して濁っている場合は、蒸留などによって精製した後、速やかに用いる必要があります。
性質
アクロレインは、分子内に炭素-炭素二重結合とアルデヒド基を持つ、最も構造が簡単な鎖式不飽和アルデヒド化合物です。CH2=CHCHOと表され、分子量は56.07、常温では無色または淡黄色液体であり、密度は 0.8389 (20 ℃) です。また、刺激臭を呈します。
反応性が高く、容易に重合して樹脂状の固体となります。特に光、アルカリ、強酸の存在下において不安定です。空気中でも酸化や重合が進行するため、などの重合禁止剤が安定剤としてしばしば用いられます。
解説
C3H4O(56.06).CH2=CHCHO.アクロレインともいう.もっとも低級な不飽和アルデヒドで,油脂の熱分解によって生成し,実験室的にはグリセリンを硫酸水素カリウム,硫酸マグネシウムなどを用いて脱水すると得られる.工業的にはMo-Bi酸化物に各種酸化物を添加した多元系酸化物触媒を用いて,プロペンを気相酸化すると得られる.刺激臭を有する無色の液体.融点-87 ℃,沸点52 ℃.d204 0.8410.n20D 1.3998.多量の水に可溶,エタノール,エーテルに易溶.アクリルアルデヒドをさらに多元系Mo-Ⅴ酸化物触媒を用いて気相酸化するとアクリル酸が合成される.アクリル樹脂原料のアクリル酸メチル合成の中間生成物(反応中間体)として重要である.このほか,医薬メチオニンの合成原料,グリセリンの合成原料にも用いられる.[CAS 107-02-8]
森北出版「化学辞典(第2版)
用途
アクリル酸、アクリル酸低級アルキルエステル、DL-メチオニン、2-ヒドロキンアジプアルデヒド、1,2,6-ヘキサントリオール、リジン、グルタールアルデヒド、アリルアルコールの中間原料
刺激性
アクロレインは,非常に毒性が強く、目や鼻の粘膜、呼吸器、皮膚を刺激する。油脂やプラスチックなどが不完全燃焼したときに発生するほか、タバコの煙やディーゼルエンジンの排気ガスにも含まれる。空気中では容易に酸化され、光、空気、過酸化物により重合して樹脂状物質に変化するので、保存する際は空気を断って少量のポリフェノールを加えておく。
反応性
アクロレインは,還元するとプロピオンアルデヒドを経てプロピルアルコール(1-プロパノール)を生成し、酸化するとアクリル酸になる。臭素を作用させると容易に二重結合に付加して2,3-ジブロモプロピオンアルデヒドになる。アルデヒド基-CHOをもっているので、オキシムの生成、銀鏡反応を行う。
合成
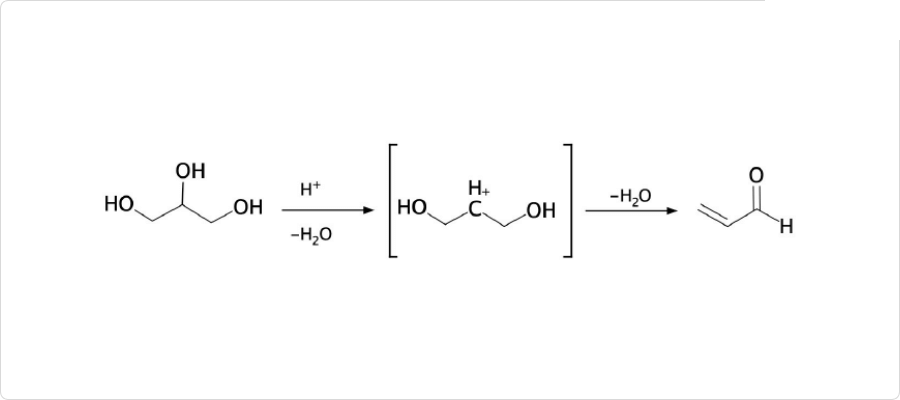
図2. アクロレインの一般的な合成方法
アクロレインは、実験室的合成法では、グリセリンをなどの脱水剤を用いて脱水することで合成されます。一般的な工業的製法は、グリセリンの高温の蒸気をに通じる合成方法です。
説明
The first time that acrolein was produced as a commercial
product was in the 1930s through the vapor-phase condensation
of acetaldehyde and formaldehyde. Another method was
developed in the 1940s, which involved the vapor-phase
oxidation of propylene. In the 1960s, some advances were
found in propylene oxidation process by the introduction of
bismuth molybdate-based catalysis, and that became the
primary method used for the commercial production of acrolein.
Some bioproducts formed for this reaction are acrylic acid,
carbon oxides, acetaldehyde, acetic acid, formaldehyde, and
polyacrolein. In World War I, it was used as a chemical weapon
(pulmonary irritant and lachrymatory agent). Commercial
acrolein contains 95.5% or more of the compound, the main
impurities being water (<3.0% by weight) and other carbonyl
compounds (<1.5% by weight), mainly propanol and acetone.
Hydroquinone is added as an inhibitor of polymerization
(0.1–0.25% by weight).
化学的特性
Acrolein is a highly flammable, clear to yellowish liquid. It has a piercing, disagreeable odor and causes tears.
物理的性質
Colorless to yellow, clear, watery liquid imparting a very sharp, acrid, pungent, or irritating odor.
Odor threshold concentrations reported were 0.11 mg/kg by Guadagni et al. (1963), 0.21 ppm
v by
Leonardos et al. (1969), and 36 ppb
v by Nagata and Takeuchi (1990). In addition, Katz and Talbert
(1930) reported an experimental detection odor threshold concentration of 4.1 mg/m
3 (1.8 ppm
v).
使用
Acrolein is used in the synthesis of acrylic acid. manufacture of colloidal forms of metals; making plastics, perfumes; warning agent in methyl chloride refrigerant. Has been used in military poison gas mixtures. Used in organic syntheses. Aquatic herbicide.
定義
A colorless liquid unsaturated aldehyde with a pungent odor. It can
be polymerized to make acrylate resins.
空気と水の反応
Highly flammable. A dangerous fire risk [Hawley]. Water soluble. Reacts slowly and exothermically with water to give 3-hydroxypropionaldehyde. A hazard can develop from this reaction if acrolein is stored over a layer of water.
反応プロフィール
ACROLEIN, [INHIBITED] can react violently with oxidizing agents. Polymerizes exothermically on contact with small amounts of acids (including sulfur dioxide), alkalis, volatile amines and pyridines, salts, thiourea, oxidizing agents (air) and on exposure to light and heat. Polymerization initiated by amines and pyridines occurs after a deceptive induction period. Water solutions of mineral acids and metal ions can initiate polymerization. The inhibitor (usually hydroquinone) greatly reduces tendency to polymerize. Undergoes Diels-Alder reaction with itself to give acrolein dimer. This can become a runaway reaction at 90°C [Kirk-Othmer, 4th Ed, Vol. 1]. Mixing in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: 2-aminoethanol, ammonium hydroxide, chlorosulfonic acid, ethylenediamine, ethyleneimine [NFPA 1991].
火災危険
Under fire conditions, polymerization may occur. If inside a container, violent rupture of the container may take place. When heated to decomposition, Acrolein emits highly toxic fumes. Alkalis or strong acids act as catalysts, causing a condensation reaction and liberating energy. Reaction may be very rapid and violent. Readily converted by oxygen to hazardous peroxides and acids. Unstable, avoid exposure to alkalis, strong acids, oxygen, elevated temperatures, such as fire conditions. (Polymerization inside container could cause violent rupture of container under fire conditions.)
燃焼性と爆発性
Acrolein is a highly flammable liquid (NFPA rating = 3) and its vapor can travel a
considerable distance and "flash back." Acrolein vapor forms explosive mixtures
with air at concentrations of 2.8 to 31% (by volume). Carbon dioxide or dry
chemical extinguishers should be used for acrolein fires.
関連する反応
アクロレインの化学反応
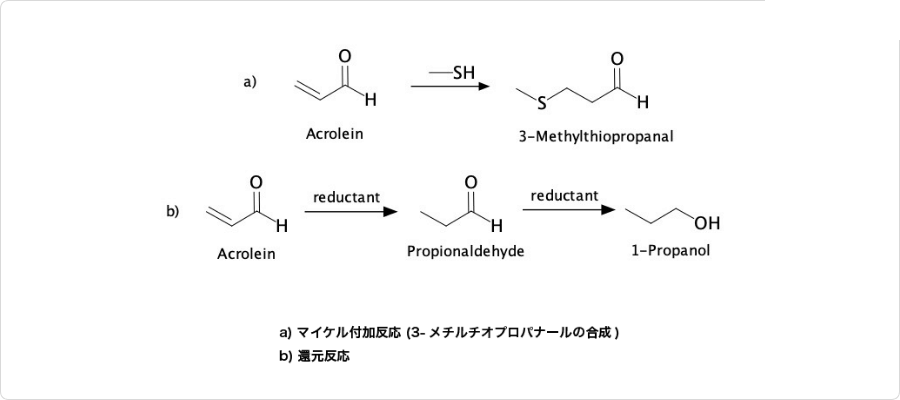
図3. アクロレインの反応の例
アクロレインは、求核剤の1,4-付加を受ける、α,β-不飽和カルボニル化合物です。マイケル付加反応のアクセプターとして、多くの反応の例があります。一例として、メチオニンの合成過程における、3-メチルチオプロパナールの生成反応が挙げられます。
尚、還元するとを経てプロパノールを生成します。これは、アルデヒド基より先にオレフィン部分が還元されるためです。
使用用途
アクロレインは、やの合成原料として使用されます。また、繊維処理剤や架橋結合剤などのほか、メチオニンなどの医薬品合成時の原料にも使われています。コロイド状オスミウ ム、、の製造、溶剤、抽出も用途の一つです。
アクロレインは、塗料に含まれる樹脂成分にも使用されていた歴史を持ちますが、現在はが主流になったことから、ほとんど使用されていません。
また、人体においては脳梗塞の発症時に血液中にアクロレインが増加することが知られています。脳梗塞においては、脳の血管が詰まり、周囲の細胞が壊れます。この際に、細胞から漏出したポリアミンがポリアミンオキシダーゼにより分解され、その結果、代謝物としてアクロレインが血中に増加するのです。そのため、アクロレインは、脳梗塞のリスク (無症状性脳梗塞) の検出を行うバイオマーカーとして、注目されています。
安全性プロファイル
Human poison by inhalation and intradermal routes. Poison experimentally by most routes. Human systemic irritant and pulmonary system effects by inhalation include: lachrymation, delayed hypersensitivity with multiple organ involvement, and respiratory system damage. Severe eye and skin irritant. Experimental reproductive effects. Human mutation data reported. Questionable carcinogen. Dangerous fire hazard when exposed to heat, flame, or oxidizers. An explosion hazard. Incompatible with amines, SO2, metal salts, oxidants, (light + heat). Violent polymerization reaction on contact with strong acid, strong base, weak acid conditions (e.g., nitrous fumes, sulfur dioxide, carbon dioxide), thiourea, or dimethylamine. When heated to decomposition it emits highly toxic fumes; can react vigorously with oxidizing materials. To fight fire, use CO2, dry chemical, or alcohol foam,
職業ばく露
Used as pharmaceutical; slimicide; and in production of cosmetics and food supplements; as an intermediate in the production of glycerine and in the production of methionine analogs (poultry feed protein supplements). It is also used in chemical synthesis (1,3,6-hexametriol and glutaraldehyde); as a liquid fuel; antimicrobial agent, in algae and aquatic weed control; and as a slimicide in paper manufacture; making plastics, drugs, and tear gas. Also, most allyl compounds may be metabolized to allyl alcohol which is metabolized to acrolein.
発がん性
Acrolein is a reactive intermediate
of the commonly used chemotherapeutic drugs cyclophosphamide
and ifosphamide. Acrolein-modified
DNA was found in human peripheral blood lymphocytes
from cancer patients previously treated with cyclophosphamide
(a chemotherapeutic), but no association was
found for cyclophosphamine. Acrolein has a classification
of C, possible human carcinogen, based on limited
animal carcinogenicity data and paucity of human evidence
for this effect.
代謝経路
When fish are exposed to 14C-acrolein, the
metabolites are identified from the edible tissues and
there is very little similarity in the metabolism of
acrolein among the test species. The most notable
observation is that acrolein is never detected in any
tissues sampled, and glycidol, glycerol, 1,3-
propanediol, and glyceric acid are the major
metabolites found in catfish, crayfish, bluegill, and
clams, respectively.
貯蔵
Work with acrolein
should be conducted in a fume hood to prevent exposure by inhalation, and splash
goggles and butyl rubber gloves should be worn at all times to prevent eye and skin
contact. Acrolein should be used only in areas free of ignition sources. Containers of
acrolein should be stored in secondary containers in areas separate from amines,
oxidizers, acids, and bases.
輸送方法
Acrolein, stabilized, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquids. Inhalation Hazard Zone A.
純化方法
Purify acrolein by fractional distillation, under nitrogen, drying with anhydrous CaSO4 and then distilling under vacuum. Blacet, Young and Roof [J Am Chem Soc 59 608 1937] distilled it under nitrogen through a 90cm column packed with glass rings. To avoid formation of diacryl, the vapour is passed through an ice-cooled condenser into a receiver cooled in an ice-salt mixture and containing 0.5g catechol. The acrolein is then distilled twice from anhydrous CuSO4 at low pressure, catechol being placed in the distilling flask and the receiver to avoid polymerization. [Alternatively, hydroquinone (1% of the final solution) can be used.] [Beilstein 1 IV 3435.]
不和合性
May form explosive mixture with air. Elevated temperatures or sunlight may cause explosive polymerization. A strong reducing agent; reacts violently with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Polymerizes exothermically on contact with small amounts of acids (including sulfur dioxide), alkalis, volatile amines and pyridines, salts, thiourea, oxidizing agents (air) and on exposure to light, and heat. Polymerization initiated by amines and pyridines occurs after a deceptive induction period. Water solutions of mineral acids and metal ions can initiate polymerization. The inhibitor (usually hydroquinone) greatly reduces tendency to polymerize. Reacts with acids, alkalis, ammonia, amines, oxygenperoxides. Shock-sensitive peroxides or acids may be formed over time. Attacks zinc and cadmium
廃棄物の処理
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration. Conditions are 816 C, 0.5 second minimum for primary combustion; 1093 C, 1.0 second for secondary combustion.
アクロレイン (モノマー) 上流と下流の製品情報
原材料
準備製品