アセチルアセトン 化学特性,用途語,生産方法
外観
無色~わずかにうすい黄色, 澄明の液体
種類
アセチルアセトンは、実験室用化学試薬として市販されています。容量には、25mL、100mL、500mLなどがあります。常温試薬ですが、暗所保存が原則です。
また、アセチルアセトンの各種金属錯体 (Al, Cr, Co, VO, Cu, Fe, Ni, Zn, Zr, Sn, Ti, Inなど) は、実験室用の試薬スケールから、工業用の5kg、10kgスケールまで様々な製品が販売されています。
性質
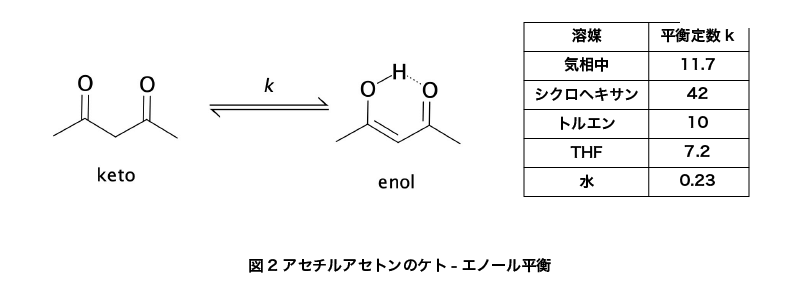
アセチルアセトンは、示性式CH3COCH2COCH3で表されます。分子量100.12、融点-23℃、沸点約141℃、引火点39℃の有機化合物です。常温では密度0.98g/mLの無色透明の液体であり、水への溶解度は16g/100mLです。
1,3ジケトンであるため、ケト-エノール平衡状態を取ります。更に、エノール体はC2v対称分子として存在していて、エノールの水素原子はちょうど2つの酸素の中間に位置して安定化を受けています。このことは、マイクロ波分光法などにより証明されました。
溶解性
水に可溶 (1 : 8水に可溶), エタノール, 他各種溶剤に易溶。エタノール、エーテルと自由に混和する。水に可溶。エタノール及びジエチルエーテルに極めて溶けやすく、水に溶けやすい。
解説
アセチルアセトン,ナトリウムまたはナトリウムアルコキシドの存在下でアセトンと酢酸エチルとをクライゼン縮合させるか,または三フッ化ホウ素の存在下にアセトンと無水酢酸を縮合させることによって得られる.無色の可燃性液体.融点-23 ℃,沸点140 ℃.d254 0.9721.n17D 1.454.希塩酸や有機溶媒に可溶.代表的なβ-ジケトンで,アセト酢酸エチルなどと同様にケト形とエノール形の平衡混合物として存在し,その平衡は溶媒によってかわる(遊離状態では70% がエノール形).多くの金属とキレートをつくるので,各種金属の抽出試薬として使われ,またキレートのなかには接触還元触媒能をもつものが多い.酸化により酢酸を生成し,アルカリ分解するとアセトンと酢酸になる.溶剤,殺虫殺菌剤,そのほか合成中間体など,工業的用途も多い.
用途
触媒 (金属キレート) 原料、接着剤原料、溶剤、有機合成中間体 (化学工業日報社)
合成
アセチルアセトンは、工業的には、酢酸イソプロペニルの熱転位によって製造されています。
実験室的合成法としては、
- を触媒として用い、アセトンとを反応させる方法
- アセトンとをアルカリ触媒によって縮合し、生成物をプロトン化する方法
などの合成方法が挙げられます。
説明
Acetylacetone (2,4-pentanedione) is a clear or slightly yellowish liquid with a putrid
odour. It is readily soluble in water and in organic solvents and incompatible with light,
ignition sources, excess heat, oxidising agents, strong reducing agents, and strong bases. On decomposition, acetylacetone releases hazardous products such as carbon monoxide,
irritating and toxic fumes and gases, and carbon dioxide. Acetylacetone is used in the
production of anti-corrosion agents and its peroxide compounds for the radical initiator
application for polymerisation. It is used as a chemical intermediate for drugs (such as
sulphamethazine, nicarbazine, vitamin B6, and vitamin K) and pesticides sulfonylurea
herbicides and pesticides. It is used as an indicator for the complexometric titration of Fe
(III), for the modification of guanidino groups and amino groups in proteins, and for the
preparation of metal acetylacetonates for catalyst application.
化学的特性
Acetylacetone (2,4-pentanedione) is a clear or slightly yellowish liquid with a putrid
odor. It is readily soluble in water. It is with other incompatible materials, light, ignition
sources, excess heat, oxidizing agents, strong reducing agents, and strong bases. On
decomposition, acetylacetone releases hazardous products, such as carbon monoxide,
irritating and toxic fumes and gases, and carbon dioxide. Acetylacetone is used in the
production of anticorrosion agents and its peroxide compounds for the radical initiator
application for polymerization. It is used as a chemical intermediate for drugs (such as
sulfamethazine, nicarbazine, vitamin B6, and vitamin K), sulfonylurea herbicides, and
pesticides. It is used as a solvent for cellulose acetate, as an additive in gasoline and
lubricant, as a dryer of paint and varnish. It is used as an indicator for the complexometric
titration of Fe(III), for the modifi cation of guanidino groups and amino groups in
proteins, and in the preparation of metal acetylacetonates for catalyst application.
使用
Acetylacetone was used in preparing Y
20
3, La
20
3 and La
2CuO
4 thin films and the titanate/anatase dual-phase photocatalyst.
定義
ChEBI: A beta-diketone that is pentane in which the hydrogens at positions 2 and 4 are replaced by oxo groups.
調製方法
2,4-Pentanedione is produced by thermal or metal-catalyzed rearrangement of isopropenyl acetate(obtained from acetone and ketene):
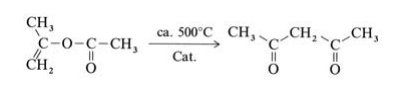
Isopropenyl acetate vapor is fed at atmospheric pressure through a V2A steel tube with an inner temperature of 520℃. The hot reaction gases are quenched, condensed, and cooled to 20℃, whereby the gaseous byproducts carbon monoxide, carbon dioxide, methane, and ketene are separated. The product is purified by fractional distillation. Other industrially less important processes for the production of 2,4-pentanedione, include the Claisen ester condensation of ethyl acetate with acetone using sodium ethoxide as condensation agent and the acetylation of acetoacetic acid esters with acetic anhydride in the presence of magnesium salts.
主な応用
Acetylacetone, also known as 2,4-pentanedione, is an important commodity chemical and widely used as a fuel additive, as dyeing intermediate, in the fields of metal extraction, metal plating, and resin modification. Hantzsch reaction was used as a derivatizing agent for the assay of compounds having a primary amino group. The reagent was reacted with the primary amino group of the drugs to form a product having color and/or emit fluorescence. This condensation reaction was distinguished by its precision, reproducibility, and analytical cost reduction. FLX contains an aliphatic amino group, in the presence of formaldehyde solution, this amino group can condense with two equivalents of acetylacetone to form dihydropyridine derivative that emits yellow fluorescent product. (Figure1). Under optimized conditions of the reaction, FLX gave highly fluorescent product measured at λem 479 nm using 419 nm as excitation.
一般的な説明
A colorless or yellow colored liquid. Less dense than water. Flash point 105°F. Vapors are heavier than air. Used as a solvent in paints and varnishes.
空気と水の反応
Flammable. Soluble in water.
反応プロフィール
Ketones, such as 2,4-Pentanedione, are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4. May dissolve plastics [USCG, 1999].
健康ハザード
Inhalation causes dizziness, headache, nausea, vomiting and loss of consciousness. Contact with liquid irritates eyes.
火災危険
Behavior in Fire: Vapor is heavier than air and may travel to a source of ignition and flash back.
使用用途
アセチルアセトンは、金属イオンの抽出剤として使用することが可能です。理由として、アセチルアセトンの共役塩基アセチルアセトナート (略号 acac) は、2つの酸素原子を介して二座配位子として多くの遷移金属イオンと六員環結合を形成することが挙げられます。
また、アセチルアセトンの金属錯体には、広範囲にわたる使用用途があります。具体的には、触媒や反応試薬の、NMRシフト試薬、有機合成における遷移金属触媒や、工業的なヒドロホルミル化触媒の前駆体などです。
その他にも、アセチルアセトンは、ガソリンや潤滑油の添加剤としても知られています。近年では、色素増感型太陽電池の開発において、ベースとなる酸化チタン (Ⅳ) にアセチルアセトンを添加すると性能が向上するとの報告があります。
安全性プロファイル
Poison by ingestion and intraperitoneal routes. Moderately toxic by inhalation. A skin and severe eye irritant. Experimental reproductive effects. Mutation data reported. Flammable liquid when exposed to heat or flame. Incompatible with oxidning materials. To fight fire, use alcohol foam, CO2, dry chemical.
職業ばく露
Acetoacetic acid derivative. 2,4-Pentanedione is used in gasoline and lubricant additives, fungicides, insecticides, and colors manufacture; as a chemical intermediate and in the manufacture of metal chelates
貯蔵
Acetylacetone should be stored away from heat, sparks, flame, and from sources of ignition.
It should be stored in a tightly sealed container, in a cool, dry, well-ventilated area,
away from incompatible substances.
輸送方法
UN2310 Pentane-2,4-dione, Hazard Class: 3; Labels: 3-Flammable liquid
純化方法
Small amounts of acetic acid are removed by shaking with small portions of 2M NaOH until the aqueous phase remains faintly alkaline. The sample, after washing with water, is dried with anhydrous Na2SO4, and distilled through a modified Vigreux column (p 11) Cartledge J Am Chem Soc 73 4416 1951]. An additional purification step is fractional crystallisation from the liquid. Alternatively, there is less loss of acetylacetone if it is dissolved in four volumes of *benzene and the solution is shaken three times with an equal volume of distilled water (to extract acetic acid): the *benzene is then removed by distillation at 43-53o and 20-30mm through a helices-packed column. It is then refluxed over P2O5 (10g/L) and fractionally distilled under reduced pressure. The distillate (sp conductivity 4 x 10-8 ohm-1cm-1) is suitable for polarography [Fujinaga & Lee Talanta 24 395 1977]. To recover used acetylacetone, metal ions are stripped from the solution at pH 1 (using 100mL 0.1M H2SO4/L of acetylacetone). The acetylacetone is then washed with (1:10) ammonia solution (100mL/L) and with distilled water (100mL/L, twice), then treated as above. It complexes with Al, Be, Ca, Cd, Ce , Cu, Fe2+, Fe3+ , Mn, Mg, Ni, Pb and Zn. [Beilstein 1 H 777, 1 I 401, 1 II 831, 1 III 3113, 1 IV 3662.]
不和合性
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. reducing agents; halogens, aliphatic amines; alkanolamines, organic acids; isocyanates. Strong light may cause polymerization.
廃棄物の処理
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
予防処置
Occupational workers should only use/handle acetyl acetone in a well-ventilated area,
with spark-proof tools and explosion-proof equipment. Workers should not cut, weld,
braze, solder, drill, grind, pressurize, or expose empty containers to heat, sparks, or
flames.
アセチルアセトン 上流と下流の製品情報
原材料
準備製品
テトラヒドロジフェルロイルメタン
4-メトキシメチル-6-メチル-2-オキソ-1,2-ジヒドロ-3-ピリジンカルボニトリル
トリス(2,4-ペンタンジオナト)アルミニウム(III)
17-Ethinyl-3,17-dihydroxy-18-methylestra-2,5(10)-diene3-methylether
ニンヒドリン
3-(3,5-ジメチル-1H-ピラゾール-1-イル)プロパン-1-アミン
1,2-DIHYDRO-4-(METHOXYMETHYL)-6-METHYL-5-NITRO-2-OXONICOTINONITRILE
4-methoxymethylpyridoxine
2-アミノ-4,6-ジメチルピリジン-3-カルボオキサミド
5-BROMO-2-CHLORO-4,6-DIMETHYLNICOTINONITRILE
4,6-ジメチル-2-(メチルチオ)ピリミジン
4,6-DIMETHYL-PYRIMIDINE-2-SULFONYL FLUORIDE
1,4,6-Trimethyl-1H-pyrazolo[3,4-b]pyridin-3-ylamine ,97%
2-(2-CARBOXYETHYL)THIO-4,6-DIMETHYLPYRIMIDINE
4,6-DIMETHYL-2-THIOPYRIMIDINE
(4,6-ジメチルピリミジン-2-イル)チオ酢酸
13-Ethyl-17-hydroxy-18,19-dinorpregn-5(10)-en-20-yn-3-one
5-アセチル-2,4-ジメチルチアゾール
ビス(2,4-ペンタンジオナト)パラジウム(II)
4,6-ジメチル-2-ヒドロキシピリミジン 塩酸塩
1,2-ジヒドロ-4,6-ジメチル-2-オキソ-3-ピリジンカルボニトリル
2-エチル-3-メチルピラジン
MEQUINDOX
2-クロロ-4-(メトキシメチル)-5-ニトロ-6-メチルピリジン-3-カルボニトリル
17-Ethinyl-17-hydroxy-18-methylestra-5(10),9(11)-dien-3-one-3-ethylene ketal
2-アミノ-4,6-ジメチルピリミジン
1-(4-METHYL-2-(METHYLTHIO)PYRIMIDIN-5-YL)ETHANONE
3-(PYRIMIDIN-2-YLTHIO)PENTANE-2,4-DIONE
1-(2,4-DIMETHYLQUINOLIN-3-YL)ETHANONE HYDROCHLORIDE
2-クロロ-4,6-ジメチルピリジン-3-カルボニトリル
3,5-ジメチル-1-ピラゾリルホルムアミジニウム硝酸塩
5-AMINO-4-(METHOXYMETHYL)-6-METHYL-3-PYRIDINEMETHANAMINE
3,5-ジメチル-1H-ピラゾール-4-カルボン酸
スルファメサジン
3-ACETYL-2-METHYL-QUINOLINE-4-CARBOXYLIC ACID
5-ブロモ-4,6-ジメチル-1H-ピラゾロ[3,4-B]ピリジン-3-アミン
4,6-ジメチル-2-ヒドロキシピリジン
17-Ethynyl-18-methylestra-5(10),9(11)-dien-17-ol-3-one
4,6-ジメチル-1H-ピラゾロ[3,4-B]ピリジン-3-イルアミン
2-CHLORO-3-(3,5-DIMETHYL-PYRAZOL-1-YL)-QUINOXALINE