イソトニタゼン 化学特性,用途語,生産方法
説明
Isotonitazene is a potent synthetic opioid, and it is being abused for its opioidergic effects. The abuse of isotonitazene, similar to other synthetic opioids, has been associated with adverse health effects, including numerous deaths. The availability of synthetic opioids in the illicit drug market continues to pose an imminent hazard to the public safety. Adverse health effects associated with the abuse of synthetic opioids and the continued evolution and increased popularity of these substances have been a serious concern in recent years. As the United States continues to experience an unprecedented epidemic of opioid misuse and abuse, the presence of new synthetic opioids with no approved medical use exacerbates the epidemic. Beginning in April 2019, isotonitazene emerged on the illicit synthetic drug market as evidenced by its identification in drug seizures.
化学的特性
Isotonitazene is chemically known as, N,N-diethyl-2-(2-(4-isopropoxybenzyl)-5-nitro-1H-benzimidazol-1-yl)ethan-1-amine. Chemical syntheses of isotonitazene and other schedule I synthetic opioid substances containing a benzimidazole core, such as etonitazene and clonitazene, were first reported in the late 1950s.
物理的性質
Both the free base and salts of isotonitazene are solids.
The measured melting point for isotonitazene hydrochloride salt is 172–173 °C (Hoffmann et al., 1960; Hunger et al., 1960b). Isotonitazene is lipophilic (calculated logP = 4.85).
Isotonitazene, as both the freebase and the hydrochloride salt, is soluble in methanol (NPS Discovery, 2019; Blanckaert et al., 2020) and in dimethyl sulfoxide (DMSO) (Blanckaert et al., 2020). Although no experimental data are available, the salts of isotonitazene, similar to etonitazene, are expected to be sufficiently water-soluble for injectable administration of effective doses.
To date, seizures and collected samples containing isotonitazene reported to the EMCDDA have been in brown, yellow, and white powders. In addition, isotonitazene has also been identified in liquid form. Identifications of isotonitazene reported to the EMCDDA include both the free base and the hydrochloride salt.
EMCDDA technical report on the new psychoactive substance N,N-diethyl-2-[[4-(1-methylethoxy)phenyl]methyl]-5-nitro-1H- benzimidazole-1-ethanamine (isotonitazene)
来歴
Isotonitazene, also called “iso,” is a designer drug that was first made decades ago as a pain relief treatment, like many other synthetic opioids. The drug is derived from a powerful opioid called etonitazene, which gives it both its pain-relieving properties and the “high” that users seek. Despite the fact that it has been around for years, the World Health Organization never approved it for marketing due to its addictive properties and risk of side effects like respiratory failure, which is side effect of many opioid pain relievers. But that hasn’t stopped the spread of this designer drug.
Though synthetic opioids, such as fentanyl, have gained popularity across the United States, isotonitazene poses a unique problem because it has not yet been officially added to the U.S. Drug Enforcement Administration’s (DEA) list of controlled substances. This has made it harder to track cases of isotonitazene overdoses, since it does not show up on most screenings.
While it was not approved for legal medical use around the world, isotonitazene was never banned by the DEA. This means that it was easier for the drug to be produced and sold in bordering countries and, eventually, the United States. As of now, the DEA and Department of Justice have declared an emergency and temporary placement of isotonitazene as a Schedule I drug. This means that it has been placed among the substances most likely to lead to abuse.
使用
Isotonitazene is an analytical reference material categorized as an opioid.Isotonitazene is regulated as a Schedule I compound in the United States. This product is intended for research and forensic applications.
製造方法
The synthesis of isotonitazene is specifically described in a patent and is outlined in Figure 1 (Hoffmann et al., 1960).The activated chloro atom in the readily available 1-chloro-2,4-dinitrobenzene is easily displaced by 2- diethylaminoethylamine. Regioselective reduction of the nitro function adjacent to the alkylamino moiety of the resultant 2,4-dinitroaniline derivative is accomplished using ammonium sulfide. Condensation of the obtained ortho-phenylenediamine species (12) with the imidate of 4- isopropoxyphenylacetic acid, obtained from the corresponding cyanide, affords the final product, that is isotonitazene. Purification is accomplished by base-acid extraction followed by conversion of the free base into its hydrochloride salt.
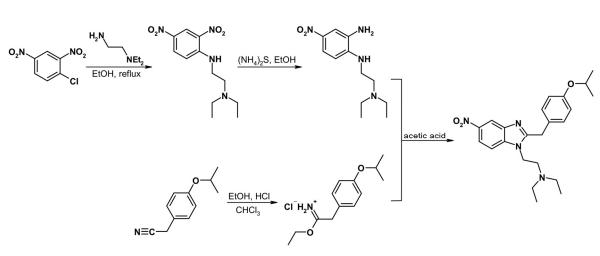
Figure 1: synthesis of isotonitazene
薬理学
Data obtained from pre-clinical studies demonstrate that isotonitazene exhibits a pharmacological profile similar to that of etonitazene and other mu-opioid receptor agonists. In an in vivo (in mice) study, isotonitazene was more potent than morphine as an analgesic in a tail-flick assay. Data from in vitro studies showed that isotonitazene, similar to fentanyl and hydromorphone, activates the mu-opioid receptors. Activation of the mu-opioid receptors by isotonitazene has been reported to involve recruitment of β-arrestin-2, a regulatory protein. Studies have shown that mu-opioid receptor and β-arrestin-2 interaction is implicated in some of the adverse effects of opioid analgesics. Naloxone, an opioid receptor antagonist, blocked isotonitazene’s activation of the mu-opioid receptor. These data demonstrate that isotonitazene, similar to fentanyl and other mu-opioid receptor agonists, binds to, and activates the mu-opioid receptor.
It is well established that substances that act as mu-opioid receptor agonists have a high potential for addiction and can cause dose-dependent respiratory depression. In fact, the abuse of isotonitazene has been associated with at least 49 fatalities in the United States.
法令条例
Isotonitazene is not an approved pharmaceutical product and is not approved for medical use anywhere in the world. DEA has issued a temporary order placing isotonitazene into Schedule I of the CSA (85 FR 51342). Prior to the temporary order, under 21 U.S.C. § 802 (32)(A), isotonitazene can be treated as an analogue of etonitazene, a schedule I substance.
イソトニタゼン 上流と下流の製品情報
原材料
準備製品