ビフェニル 化学特性,用途語,生産方法
外観
白色~ほとんど白色、 結晶~結晶性粉末又はフレーク
性質
ビフェニルは,ベンゼン系芳香族炭化水素の一つ。ジフェニルともいう。 二つのフェニル基が直接に炭素‐炭素(C-C)結合した構造であるが、2個のフェニル基-C6H5は同じ平面上になく、42度の角でねじれている。芳香をもつ無色の結晶。ヨードベンゼンと銅粉を加熱して合成される(ウルマンの合成法)。このほか臭化ベンゼンをベンゼン中で光分解すると生成する。伝熱媒体として用いられるほか、染料、医薬品などの合成原料としての用途がある。融点71℃,沸点255.25℃。水に不溶。ヨードベンゼン類を銅粉とともに加熱し合成。加熱溶媒,有機合成原料。ビフェニルの水素を塩素で置換したものがPCBである。[向井利夫]
溶解性
水に不溶。エーテル, 芳香族炭化水素溶剤に可溶。水に不溶, ほとんどの有機溶媒に可溶水にほとんど不溶。エタノール, ジエチルエーテル, 他の有機溶媒に易溶。エタノール及びアセトンに溶け、水にほとんど溶けない。
解説
1,1′-biphenyl.C12H10(154.20).C6H5-C6H5.ジフェニルともいう.コールタール中に少量含まれるが,ベンゼンの蒸気を赤熱した鉄管中に通じれば生成する.また,ヨードベンゼン 2分子を銅粉を用いて,ウルマン反応により縮合させると得られる.結晶.融点71 ℃,沸点255 ℃.nD77"1.588.λmax 206,247 nm(log ε 4.52,4.26).エタノール,エーテルなどに可溶.熱的に安定で熱媒体として,また,かんきつ類の殺菌剤に用いられる.LD50 3280 mg/kg(ラット,経口).[CAS 92-52-4]
森北出版「化学辞典(第2版)
用途
熱媒体,染色助剤
構造
ビフェニルは室温下の結晶においては、ような平面構造をとっていますが、固相あるいは液相、または結晶を相転移温度以下まで冷却すると、二つのベンゼン環が互いにねじれた構造をとります。
有機化合物は共役構造をとることで安定化します。単純に共役構造をとるだけであれば、各々の元素の電子軌道をできるだけ重ねるために平面構造をとることがエネルギー的に有利になりますが、ビフェニルの場合はオルト基の水素原子同士が立体障害を起こします。
つまり、「共役構造による安定化」と「水素原子の立体障害」のバランスによって、エネルギー的に有利なねじれ角度が決まります。
農薬用途
殺菌剤
使用上の注意
不活性ガス封入
説明
Biphenyl, also called diphenyl, consists of two benzene rings joined by a single bond. It exists as colorless to yellowish crystals, has a distinctive odor, and occurs naturally in oil, natural gas, and coal tar.
化学的特性
1,1'-Biphenyl is a clear colorless liquid with a pleasant odor and stable organic compound. It is combustible at high temperatures, producing carbon dioxide and water when combustion is complete. Partial combustion produces carbon monoxide, smoke, soot, and low molecular weight hydrocarbons. It is used extensively in the production of heat-transfer fl uids, for example, as an intermediate for polychlorinated biphenyls, and dye carriers for textile dyeing. It is also used as a mold retardant in citrus fruit wrappers, in the formation of plastics, optical brighteners, and hydraulic fluids.
物理的性質
White scales with a pleasant but peculiar odor. Odor threshold concentration is 0.83 ppb (quoted,
Amoore and Hautala, 1983).
天然物の起源
Reported found in coal tar, bilberry, carrots, peas, potato, bell pepper, rum, cocoa, tomato, coffee, roasted
peanuts, olive (Olea europae), buckwheat and tamarind (Tamarindus indica L).
使用
Biphenyl is used as an antifungal agent to preserve citrus fruit, in citrus wrappers to retard mold growth, in heat transfer fluids, in dye carriers for textiles and copying paper, as a solvent in pharmaceutical production, in optical brighteners, and as an intermediate for the production of a wide range of organic compounds.
製造方法
By thermal dehydrogenation of benzene.
定義
ChEBI: A benzenoid aromatic compound that consists of two benzene rings connected by a single covalent bond. Biphenyl occurs naturally in coal tar, crude oil, and natural gas. Formerly used as a fungicide for citrus crops.
安全性
ビフェニルは、眼刺激性及び発がん性が指摘されています。また、長期の暴露により肝臓、腎臓、神経系への悪影響をもたらす可能性があります。ビフェニルだけでなく、ビフェニルの誘導体も毒性が高いため、利用する際には使用環境に応じた安全設備や保護具の着用が必要です。
ビフェニルは以下の法令指定物質に指定されています。
- 労働安全衛生法 健康障害防止指針公表物質
- PRTR法 第一種指定化学物質
- 大気汚染防止法 有害大気汚染物質
一般的な説明
A clear colorless liquid with a pleasant odor. Flash point 180°F. Insoluble in water. Vapors are heavier than air. Used to manufacture other chemicals and as a fungicide.
空気と水の反応
Insoluble in water.
反応プロフィール
Biphenyl is incompatible with oxidizers.
健康ハザード
Exposures to 1,1’-biphenyl cause acute effects with symptoms that include, but are not
limited to, polyuria, accelerated breathing, lacrimation, anorexia, weight loss, muscular
weakness, coma, fatty liver cell degeneration, and severe nephrotic lesions. Exposure to
biphenyl fumes for short periods of time causes nausea, vomiting, irritation of the eyes
and respiratory tract, and bronchitis. Breathing small amounts of 1,1’-biphenyl over long
periods of time causes damage to the liver and the nervous system of exposed workers.
Breathing the mists, vapors, or fumes may irritate the nose, throat, and lungs. Depending
on the concentration and duration of exposure, the symptoms include, but are not limited
to, sore throat, coughing, labored breathing, sneezing, a burning sensation, and the effects
of CNS depression. Symptoms may include headache, excitation, euphoria, dizziness,
incoordination, drowsiness, light-headedness, blurred vision, fatigue, tremors, convulsions, loss of consciousness, coma, respiratory arrest, and death, depending on the concentration and duration of exposure
火災危険
Combustible. Emits toxic fumes under fire conditions.
使用用途
ビフェニルの主な用途として、防カビ剤、合成樹脂、熱媒体およびその原料が挙げられます。
日本では防カビ剤としての使用が多く、特に柑橘類の防カビ目的でよく使用されていましたが、ビフェニルに対する耐性菌が発見されてからはあまり使用されていません。他の使用用途としては、過充電防止剤としてのに添加されることがあります。
安全性プロファイル
Poison by intravenous route.Moderately toxic by ingestion. A powerful irritant byinhalation in humans. Human systemic effects byinhalation of very small amounts: flaccid paralysis, nauseaor vomiting, and other unspecified gastrointestinal effects.Que
職業ばく露
Biphenyl is a fungicide (pesticide). It
is also used as a heat transfer agent, dye carrier, and as an
intermediate in organic synthesis.
代謝経路
Biphenyl is a stable compound. It has a restricted use as a fungistat
applied to citrus packing material. This has generated studies on its
toxicity and metabolism in mammals and residue studies in stored fruit.
Only limited information on its environmental fate has been published.
貯蔵
1,1’-Biphenyl should be kept stored in tightly closed containers in a cool, dry, isolated, and
properly ventilated area, away from heat, sources of ignition, incompatibles, and contact
with strong oxidizers.
輸送方法
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1—Poisonous materials, Technical
Name Required.
合成方法
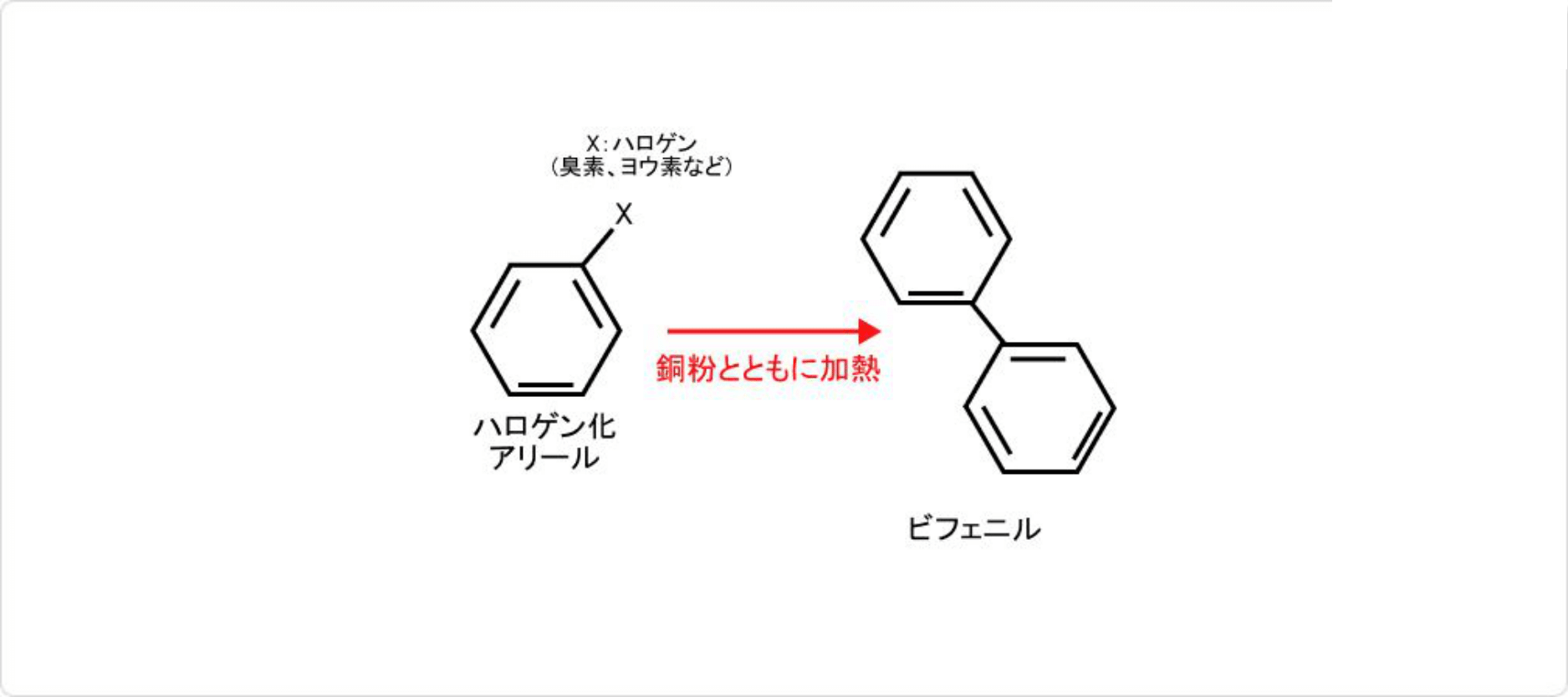
図3. ウルマン反応
ビフェニルは様々な方法で合成することができます。知られている合成法のうち、一部を以下に紹介します。
- ハロゲン化アリールと銅粉の加熱 (ウルマン反応)
- を赤熱した管に通す
- 臭化ベンゼンをベンゼン中で光分解
収率やコストの観点から、状況に応じて適切な合成法を選択することが重要です。
純化方法
Crystallise biphenyl from EtOH, MeOH, aqueous MeOH, pet ether (b 40-60o) or glacial acetic acid. Free it from polar impurities by passage through an alumina column in *benzene, followed by evaporation. The residue has been purified by distillation in a vacuum and by zone refining. Treatment with maleic anhydride removes anthracene-like impurities. It has been recrystallised from EtOH followed by repeated vacuum sublimation and passage through a zone refiner. [Taliani & Bree J Phys Chem 88 2351 1984, Beilstein 5 H 576, 5 I 271, 5 II 479, 5 III 1726, 5 IV 1807.]
不和合性
Mist may form explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides.
廃棄物の処理
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator
equipped with an afterburner and scrubber. All federal, state,
and local environmental regulations must be observed.
予防処置
Occupational workers should be careful during use and chemical management because the fi nely dispersed particles of 1,1’-biphenyl form explosive mixtures in air
ビフェニル 上流と下流の製品情報
原材料
準備製品
4-ビフェニルスルホニル クロリド
polyquinoxaline
2-フェニルフェノール
4-フェニルベンゾフェノン
4,4'-ジアセチルビフェニル
ラロキシフェン
4,4'-ビス(ブロモメチル)ビフェニル
6-bromoquinolin-4(3H)-one
エストラジオール
(R)-α-フェニル-4-メチルフェネチルアミン
フェンブフェン
4-フェニル安息香酸
ナフタレン
4-ブロモビフェニル
3-(1,1'-ビフェニル-4-イル)-1,2,3,4-テトラヒドロナフタレン-1-オール
4-(クロロフェニルメチル)-1,1'-ビフェニル
6-ブロモ-4-クロロキノリン
3-ヒドロキシメチル-2-メチルビフェニル
ブロマジオロン
4,4'-ビス(クロロメチル)ビフェニル
3-(4'-ブロモ-1,1'-ビフェニル-4-イル)-1,2,3,4-テトラヒドロナフタレン-1-オール
4-ビフェニリルアミン
4-ベンジルビフェニル
2-(クロロメチル)-4-フェニル-6-クロロキナゾリン3-オキシド
2-(アセトキシ)-3-ブロモ-5-クロロ-N-(4-ブロモフェニル)ベンゼンカルボチオアミド
2-(2-AMINO-4-BIPHENYL)PROPIONITRILE
ビホナゾール
4-フェニルフェノール
fluorescent whitening agent ATS-X
1-(4-ビフェニリルオキシ)-3,3-ジメチル-1-(1H-1,2,4-トリアゾール-1-イル)-2-ブタノール
p-ターフェニル
2-フェニルインドール
4-アセチルビフェニル