酸化ベリリウム 化学特性,用途語,生産方法
種類
酸化ベリリウムは、主に研究開発用試薬製品として販売されている他、産業用セラミック素材として販売されています。
1. 研究開発用試薬製品
研究開発用試薬製品としては、主に5g、10g、25g、100g、500gなどの容量の種類が有ります。実験室で取り扱いやすい容量での提供が中心です。安定な化合物であるため、通常室温で保管可能な試薬製品として取り扱われています。
2. 産業用セラミック素材
酸化ベリリウムは、優れた絶縁性と熱伝導性を併せ持つ産業用セラミック素材としても販売されています。様々なグレードが有り、強度と熱伝導性が異なっています。購入に当たってはメーカーへの個別の問い合わせが必要です。
性質
酸化ベリリウムは、分子量25.01、融点2,570℃、沸点3,900℃であり、常温での外観は白色粉末または無色結晶です。結晶構造は六方晶系のウルツ鉱型構造であり、ベリリウムおよび酸素原子は4配位となっています。
密度は3.02g/mL、水にはほぼ不溶です (水への溶解度: 0.2 g/1dm3) 。濃硫酸および濃塩酸との加熱によって溶解します (溶解過程の生成物: 硫酸ベリリウム或いは塩化ベリリウム)。フッ化水素酸にはフルオロ錯体を生成して溶解します。通常の保管環境においては非常に安定ですが、直射日光と高温を避けて保管することが必要です。
解説
酸化ベリリウム.ベリリウムの炭酸塩または硝酸塩を強熱すると得られる.白色の粉末.融点2570 ℃,沸点3900 ℃.密度3.02 g cm-3(0 ℃).複屈折を示す.水にきわめて難溶.フッ化水素酸に溶け,濃硫酸,濃塩酸にも加熱すると溶ける.高温でも化学的に安定である.ベリリウム塩の製造,原子炉の減速材や反射材,ロケットの先頭部や燃焼室,セラミックス,サーメット,耐火物,るつぼ,絶縁体原料,光学ガラスなどに用いられる.粉末は有毒.ベリリウムと酸素の化合物。ベリリウムの金属または化合物を空気中で燃焼すると得られるが、工業的には緑柱石(3BeO・Al2O3・6SiO2)より製造される。すなわち、原料を電気炉中で1500℃以上で融解し、水で急冷するとガラス状となる。この物質から硫酸を用いてベリリウム成分を抽出し、硫酸ベリリウムとして晶出させる。これをアルカリで処理して水酸化物としてから1100℃で熱分解する。白色の結晶性粉末。化学的に安定であるうえ、その焼結体は著しい熱伝導率を示し、絶縁抵抗、熱衝撃抵抗が大きいので、各種電子材料として、またロケットのノズルやるつぼなどの耐熱材料として用いられる。また、熱中性子に対する吸収断面積が小さく、散乱断面積が大きいため、原子炉用の減速材や反射材としての用途もある。
合成
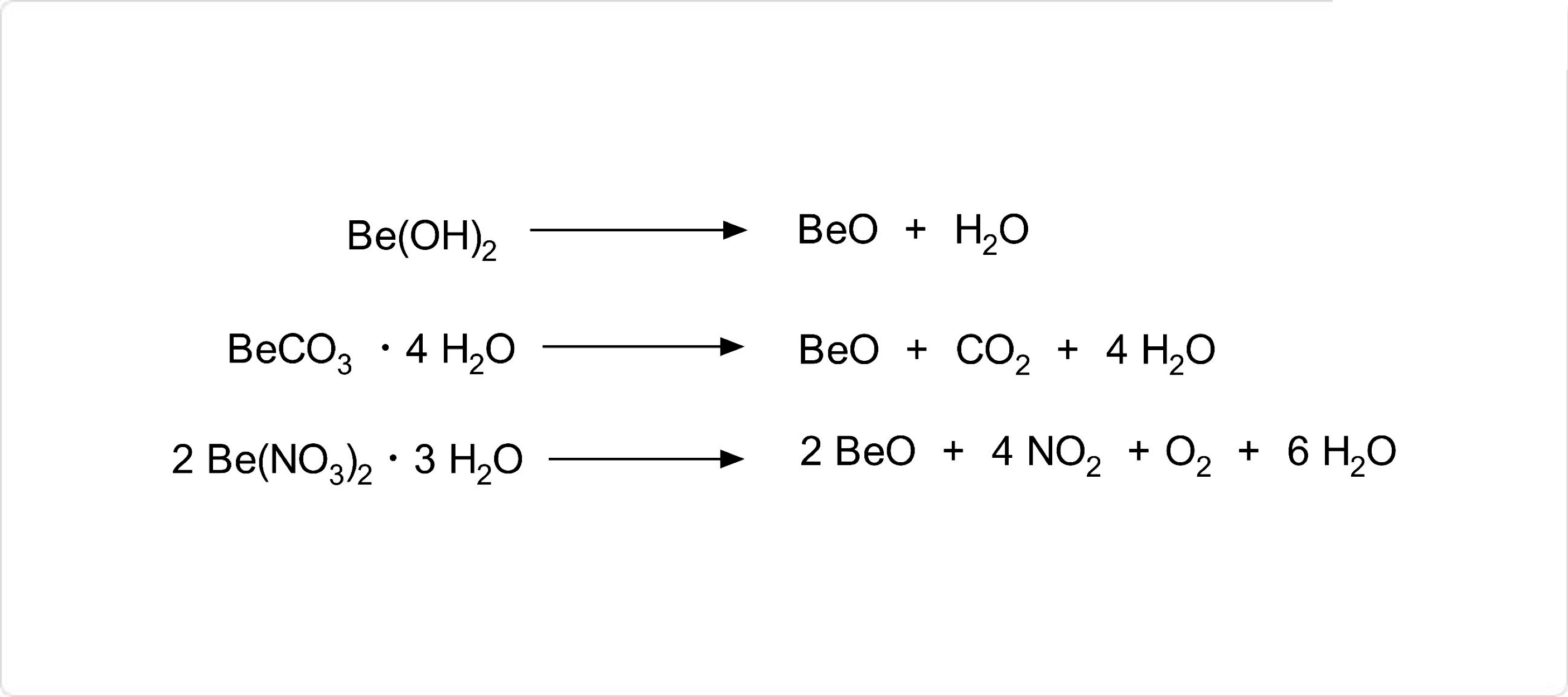
図. 酸化ベリリウムの合成
酸化ベリリウムの製造方法として、ベリリウムの炭酸塩または、ベリリウムの硝酸塩の加熱分解が挙げられます。水酸化ベリリウムの熱分解によっても酸化ベリリウムの合成は可能です。
工業的には、緑柱石を材料に酸化ベリリウムを製造する方法が一般的です。具体的には、まず、緑柱石を1,500℃で融解させた後に冷却し、状の生成物をと反応させて、中間体の硫酸ベリリウムを得ます。次にこの硫酸ベリリウムをアルカリで処理した後に加熱によって分解すると、酸化ベリリウムが生成します。
説明
Beryllium oxide
(BeO) is formed by the ignition of beryllium metal in an oxygen atmosphere. The resulting solid is colourless
and insoluble in water.
物理的性質
In the transmittance and reflectance spectra of BeO, the main peak of the reflectance is located at 730 cm
-1and the small peak is located at 1050 cm
-1.The fundamental frequency of the lattice absorption is υ
0=700±10 cm
-1, according to the reflectance analysis.
物理的性質
It is the only material with
diamond that combines both
excellent thermal shock
resistance, high electrical
resistivity, and high thermal
conductivity, and hence is used
for heat sinks in electronics.
Beryllia is very soluble in water,
but slowly in concentrated acids
and alkalis. Highly toxic.
Exhibits outstanding corrosion
resistance to liquid metals Li,
Na, Al, Ga, Pb, Ni, and Ir.
Readily attacked by molten
metals such as Be, Si, Ti, Zr, Nb,
Ta, Mo, and W. Maximum
service temperature 2400°C.
物理的性質
Beryllium oxide (BeO) is a white crystalline oxide. It occurs in nature as the mineral “Bromellite”. Historically, beryllium oxide was called glucina or glucinium oxide. It is an electrical insulator and its thermal conductivity is such that it is higher than any other nonmetal except diamond, and actually exceeds that of some metals. Its high melting point leads to its use as a refractory.

使用
Beryllium oxide is used in the manufacture of high-temperature refractory material and high-quality electrical porcelains, such as aircraft spark plugs and ultrahigh-frequency radar insulators. The high thermal conductivity of beryllium oxide and its good high- frequency electrical insulating properties find application in electrical and electronic fields.
Another use of beryllium oxide is as a slurry for coating graphite crucibles to insulate the graphite and to avoid contamination of melted alloys with carbon. Beryllium oxide crucibles are used where exceptionally high-purity or reactive metals are being melted. In the field of beryllium oxide ceramics, a type of beryllia has been developed that can be formed into custom shapes for electronic and microelectronic circuits. Beryllium oxide has a high thermal conductivity, equal to that of aluminum, and excellent insulating properties, which permits closer packing of semiconductor functions in silicon integrated circuits.
定義
ChEBI: A beryllium molecular entity consisting of beryllium (+2 oxidation state) and oxide in the ratio 1:1. In the solid state, BeO adopts the hexagonal wurtzite structure form while in the vapour phase, it is present as discrete diatomic covalent molecules.
製造方法
Beryllium oxide can be prepared by calcining beryllium carbonate, dehydrating the hydroxide or igniting the metal with oxygen gas, as shown in the following reactions:
BeCO
3→BeO+CO
2
Be(OH)
2→BeO+H
2O
2Be+O
2→2BeO
Igniting beryllium in air
一般的な説明
Odorless white solid. Sinks in water.
空気と水の反応
The amount of heat generated by hydrolysis may be large.
反応プロフィール
BERYLLIUM OXIDE is incompatible with the following: Acids, caustics, chlorinated hydrocarbons, oxidizers, molten lithium, magnesium .
危険性
Highly toxic by inhalation. Keep container
tightly closed and flush out after use.
健康ハザード
Any dramatic, unexplained weight loss should be considered as possible first indication of beryllium disease. Other symptoms include anorexia, fatigue, weakness, malaise. Inhalation causes pneumonitis, nasopharyngitis, tracheobronchitis, dyspnea, chronic cough. Contact with dust causes conjunctival inflammation of eyes and irritation of skin.
火災危険
Special Hazards of Combustion Products: Toxic BERYLLIUM OXIDE fume may form in fire.
化学反応
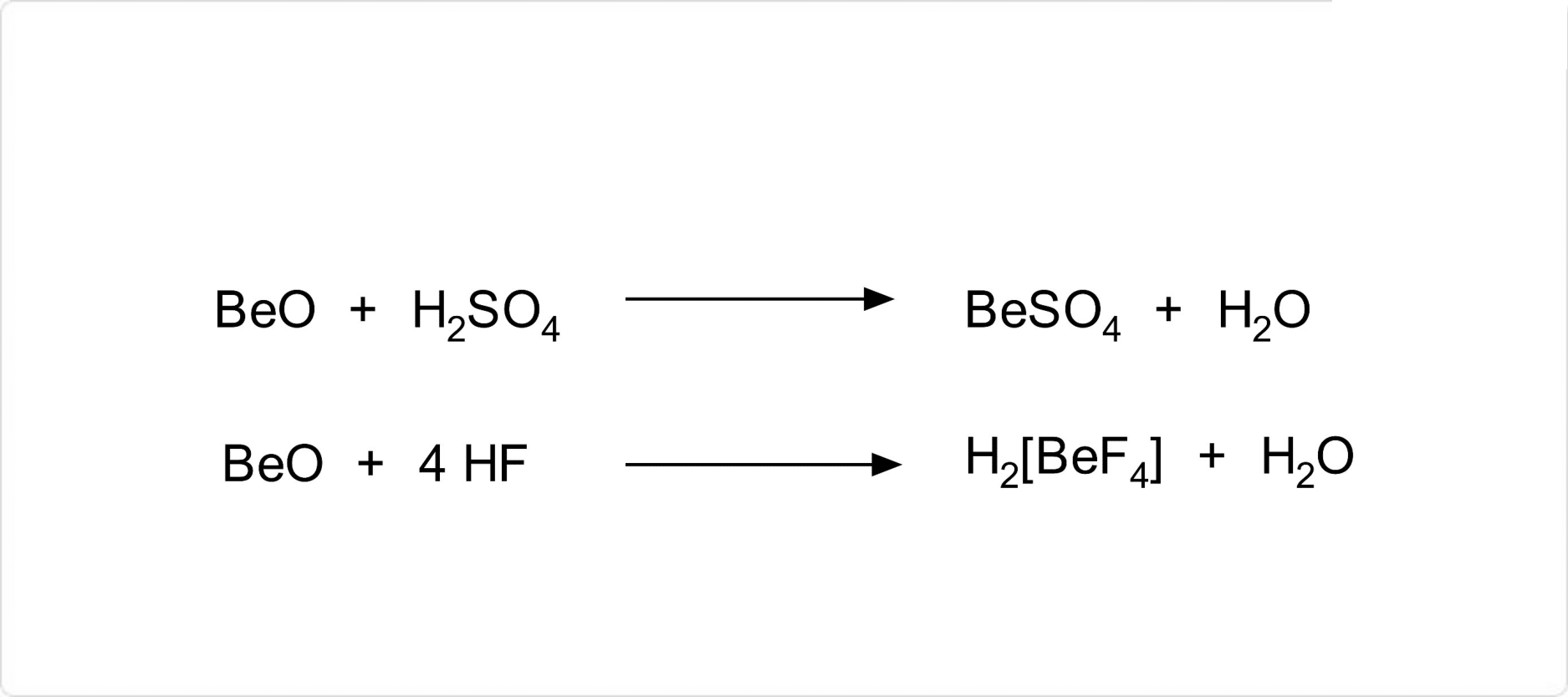
図. 酸化ベリリウムの化学反応
酸化ベリリウムは、非常に安定な化合物であり、強熱した結晶性のものは水に不溶であり、酸およびアルカリにも溶けません。ただし、濃硫酸および濃塩酸との加熱により、硫酸ベリリウムおよび塩化ベリリウムが生成します。また、フッ化水素酸を加えるとフルオロ錯体を形成して溶解します。
参考文献
使用用途
酸化ベリリウムの主な用途は、ロケットの先端部や燃料室の部品、原子炉の減速材や中性子反射材などです。様々な産業分野で幅広く使用されています。酸化ベリリウムは、化学的に非常に安定であり、沸点融点が共に高いため高温環境下でも非常に安定して存在する物質です。
これらの性質ゆえに、過酷な温度環境の分野で用いられています。そのほか、セラミック素材としても用いられます。酸化ベリリウムは熱伝導性が高いです (325W/m・K) 。セラミックの中で群を抜いているだけでなく、アルミなどの金属材料よりも優れています。
主な用途は絶縁性が必要でありながら、且つ放熱が必要な製品への使用です。具体的には、医療機器、レーザー回路基板、半導体製造装置、ガスレーザー管、半導体部品の材料などが挙げられます。
工業用途
A colorless to white crystalline powder of the composition beryllium oxide, also called beryllia. It has a specific gravity of 3.025, a high melting point, about 2585 C, and a Knoop hardness of 2000. It is used for polishing hard metals and for making hot-pressed ceramic parts. Its high heat resistance and thermal conductivity make it useful for crucibles, and its high dielectric strength makes it suitable for high-frequency insulators. Single-crystal beryllia fibers, or whiskers, have a tensile strength above 6800 MPa.
Beryllium oxide is tapped for nuclear reactor service because of its refractoriness, high thermal conductivity, and ability to moderate (slow down) fast neutrons. The thermal neutrons that result are more efficient in causing fusion of uranium- 235. Nuclear industry uses for beryllia include reflectors and the matrix material for fuel elements. When mixed with suitable nuclear poisons, beryllium oxide may be a new candidate for shielding and control rod assembly applications.
安全性プロファイル
Confirmed carcinogen withexperimental tumorigenic data. Experimental teratogenicdata. Other experimental reproductive effects. Incompatible with (Mg +heat). When heated to decomposition it emits very toxicfumes of BeO.
Structure and conformation
The space lattice of Beryllium oxide belongs to the hexagonal system with lattice constants a=0.2698 nm and c=0.4380 nm.
酸化ベリリウム 上流と下流の製品情報
原材料
準備製品