カリホルニウム 化学特性,用途語,生産方法
解説
カリホルニウム,Cf.原子番号98の元素.電子配置[Rn]5f 107s2の周期表3族アクチノイド元素.6番目に発見された超ウラン元素.実験の行われたカリフォルニア大学,カリフォルニア州にちなんで命名された.1950年,G.T. Seaborg(シーボーグ),A. Ghiorsoらにより,カリフォルニア大学(バークレー校)の60 inサイクロトロンで加速した35 MeV4He原子核(α粒子)による 242Cm の衝撃で 244Cf が得られた.逐次中性子吸収(原子炉中)で得られる.249,252Cf が利用される.密度15.1 g cm-3,融点1173 ℃ の金属.第一イオン化エネルギー608 kJ mol-1(6.3 eV).通常の酸化数2~4.CfO,Cf2O3,CfO2,CfCl3がつくられた.現在,知られている同位体核種は,237~256の範囲に20種.質量数251の核種がもっとも長寿命で半減期898 y.254Cf の半減期は当初55 d とされ,超新星の光度減衰の半減期と一致するとして宇宙での合成が考えられたが,その後60.5 d と訂正されたのでこの説は根拠が不十分と考えられている.半減期2.645 y でα崩壊(97%)および自発核分裂(3%)する 252Cf は,自発核分裂の際に中性子を発生するので,ポータブル中性子源として放射化分析による現場での金,銀探索に使われる.
説明
Californium does not occur in nature. The element was synthesized in 1949 at the Lawrence Berkeley Laboratory in Berkeley, California by Thompson, Ghiorso and Seaborg (Thompson, S.G., Ghiorso, A. and G. T. Seaborg. 1950. Phys. Rev., 77, 838). It has 12 isotopes. It is the fifth man-made transuranium element. Presently, the element has no commercial application.
化学的特性
α-form: hexagonal, a=0.339 nm, c=1.101nm; β: fcc, a=0.494nm; γ: fcc, a=0.575 nm; ionic radius of Cf+++ is 0.0934nm, of Cf+++ is 0.0851 nm; discovered in 1950; 252Cf is an intense neutron source, 1g emits 2.4×10+12 neutrons per sec; has application in neutron activation analysis and field use in mineral prospecting and oil-well logging, potential use in medical applications [KIR78]
物理的性質
Californium is a synthetic radioactive transuranic element of the actinide series. The puremetal form is not found in nature and has not been artificially produced in particle accelerators.However, a few compounds consisting of californium and nonmetals have been formedby nuclear reactions. The most important isotope of californium is Cf-252, which fissionsspontaneously while emitting free neutrons. This makes it of some use as a portable neutronsource since there are few elements that produce neutrons all by themselves. Most transuranicelements must be placed in a nuclear reactor, must go through a series of decay processes, ormust be mixed with other elements in order to give off neutrons. Cf-252 has a half-life of 2.65years, and just one microgram (0.000001 grams) of the element produces over 170 millionneutrons per minute.
Californium’s melting point is ~900°C, its boiling point is unknown, and its density is alsounknown.
同位体
There are a total of 21 isotopes of californium. None are found in nature and allare artificially produced and radioactive. Their half-lives range from 45 nanoseconds forcalifornium-246 to 898 years for californium-251, which is its most stable isotope andwhich decays into curium-247 either though spontaneous fission or by alpha decay.
名前の由来
Named for both the state of California and the University of California.
天然物の起源
Neither californium nor its compounds are found in nature. All of its isotopes are producedartificially in extremely small amounts, and all of them are extremely radioactive. All of itsisotopes are produced by the transmutation from other elements such as berkelium and americium.Following is the nuclear reaction that transmutates californium-250 into californium-252:
250Cf + (neutron and λ gamma rays) →
251Cf + (neutron and λ gamma rays) →
252Cf.
特性
Californium is a transuranic element of the actinide series that is homologous with dysprosium(
66Dy), just above it in the rare-earth lanthanide series. Cf-245 was the first isotopeof californium that was artificially produced. It has a half-life of just 44 minutes. Isotopes ofcalifornium are made by subjecting berkelium to high-energy neutrons within nuclear reactors,as follows:
249Bk + (neutrons and λ gamma rays) →
250Bk →
250Cf + β- (beta particleemission).
使用
Californium’s uses are limited, which is why the U.S. Nuclear Regulatory Commission,which controls the output and use of radioisotopes, has made californium-252 available forcommercial use at the cost of only $10 per millionth of a gram. This small quantity is adequatefor many sources of free neutrons to be used commercially. For example, free neutrons can be used in devices to measure moisture in products, including the Earth’s crust, to find wateror supplies of underground oil. Cf-252’s ability to produce neutrons has also found uses inmedicine. Cf-252’s natural spontaneous fission makes it an ideal and accurate counter forelectronic systems.
製造方法
All isotopes of the element are synthesized in the nuclear reactor. The first isotope synthesized had the mass 241, produced by irradiation of milligram quantities of americium-241 with alpha particles of 35 MeV in a cyclotron:
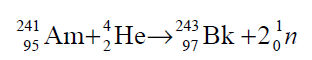
The product was separated by ion exchange
While the lighter isotopes are prepared by alpha particle bombardment, the heavier ones by neutron irradiation of large quantities of americium, curium or plutonium:
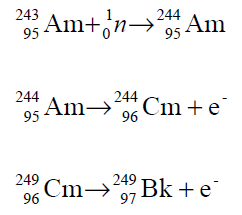
Only a small fraction of Bk-249 is obtained by the above reaction because neutrons also induce fission. Alternatively, uranium-238 may be converted to Bk-249 by very short but intense neutron bombardment followed by five successive beta decays.
定義
A silvery
radioactive transuranic element of the
actinoid series of metals, not found naturally
on Earth. Several radioisotopes have
been synthesized, including californium-
252, which is used as an intense source of
neutrons in certain types of portable detector
and in the treatment of cancer.
調製方法
Isotopes of californium may be produced in a cyclotron by neutron irradia tion or charged particle bombardment. Lighter isotopes of californium may be produced by bombardment of curium-242 or curium-244 with alpha particles having 35.5 MeV energy:
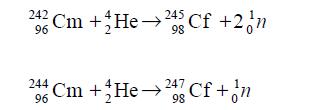
The above method was used for producing californium-245 during its first ever synthesis. Heavier isotopes of californium may be obtained by intense neutron irradiation:
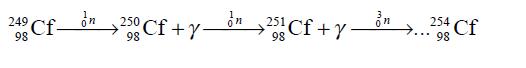
This, in turn is produced by successive slow neutron irradiation of curi um-244: Californium-254 may be produced by thermonuclear explosion resulting in the reaction of uranium-238 with intense neutron flux followed by a sequence of β- decays (Cunningham, B. B. 1968. In Encyclopedia of Chemical Elements, ed. Clifford A. Hampel, New York: Reinhold Book Co.)
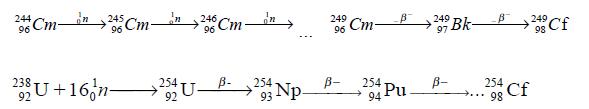
Californium is separated from other elements by fractionation and precipita tion, and further purified by solvent extraction or ion exchange.
危険性
Californium’s greatest danger is as a biological bone-seeking radioactive element, whichcan be both a radiation hazard and a useful treatment for bone cancer. If mishandled, all ofcalifornium’s isotopes and compounds can be a potential radiation poison.
カリホルニウム 上流と下流の製品情報
原材料
準備製品