ベンジルアルコール 化学特性,用途語,生産方法
外観
無色澄明の液体
定義
本品は、次の化学式で表される芳香族アルコールである。
溶解性
水に微溶 (3.5g/100ml水, 25℃), アルコール, エーテル, その他多くの有機溶剤と混和。エタノール及びジエチルエーテルに極めて溶けやすく、水にやや溶けやすい。
解説
ベンジルアルコール,天然には,ジャスミン,イランイラン,アカシアなどの多くの花精油中に遊離または酢酸,安息香酸,ケイ皮酸などのエステルの形で存在している.合成するには,塩化ベンジルを加水分解するか,ベンズアルデヒドの還元やカニッツァーロ反応によってつくることができる.芳香をもつ液体.融点-15.3 ℃,沸点206 ℃.d4151.0493.nD151.5426.アルコール系およびベンゼン系の多くの有機溶媒に可溶,水に微溶.空気中の酸素で徐々に酸化され,ベンズアルデヒドを経て安息香酸を生じる.化粧品やせっけん用の香料として用いられるほか,保留剤として,ジャスミン,その他香料の調合など,香料工業において広く使われる.局所麻酔性,防腐性があるので,注射剤の調整にも利用される.
用途
吸光分析、ケトン及びアルデヒド分析、医薬品原料。
用途
化粧品、石鹸用香料
用途
有機合成研究用、医薬品、香料及び化粧品原料。
用途
香粧品、石けん用香料として用いられ、ことにジャスミン、ガーデニア、ツベローズなどの人造花精油の調合に使用される。また保留剤、溶剤、希釈剤、エステル原料としても重要である。
用途
ベンジル誘導体原料、医薬品添加剤(殺菌剤)、溶剤、塗料?インキ?エポキシ樹脂溶剤,合成繊維染色助剤,医薬?化粧品防腐剤
化粧品の成分用途
防腐剤、減粘剤、口腔衛生剤、溶剤、外用鎮痛剤、香料
効能
抗菌性保存剤
主な用途/役割
エポキシ樹脂接着剤の希釈剤として使用される。
説明
Benzyl alcohol is a component catalyst for epoxy
resins. It is also contained in the color developer C-22.
化学的特性
Benzyl alcohol occurs in many essential oils and foods. It is a colorless liquid with a weak, slightly sweet odor. Benzyl alcohol can be oxidized to benzaldehyde, for example, with nitric acid. Dehydrogenation over a copper–magnesium oxide–pumice catalyst also leads to the aldehyde. Esterification of benzyl alcohol results in a number of important fragrance and flavor materials. Diphenylmethane is prepared by a Friedel–Crafts reaction of benzyl alcohol and benzene with aluminum chloride or concentrated sulfuric acid. By heating benzyl alcohol in the presence of strong acids or strong bases, dibenzyl ether is formed.
物理的性質
Colorless, hygroscopic, air sensitive liquid with a faint, pleasant, aromatic odor. Odor threshold
concentration in water is 10 ppm (Buttery et al., 1988).
天然物の起源
The free alcohol is often present in several essential oils and extracts of jasmine, tobacco, tea, neroli, copaiba,
Acacia farnesiana Willd., Acacia cavenia Hook. and Arn., Robinia pseudacacia, ylang-ylang, Pandanus odoratissimus, Michelia
champaca, Prunus laurocerasus, tuberose, orris, castoreum, violet leaves, clove buds and others. Also found in fresh apple, apricot,
mandarin peel oil, high bush blueberry, raspberry, strawberry fruit, American cranberry and cooked asparagus.
来歴
L
IEBIG and W
O¨HLER first prepared benzyl alcohol from bitter almond oil (benzaldehyde) in 1832. The structure of benzyl alcohol was determined in 1853 by C
ANNIZZARO. C
ANNIZZARO used the reaction named after him, in which benzaldehyde is disproportionated into benzoic acid and benzyl alcohol through the action of an alkali.
使用
benzyl alcohol is a preservative against bacteria, used in concentrations of 1 to 3 percent. It can cause skin irritation.
定義
ChEBI: Benzyl alcohol is an aromatic alcohol that consists of benzene bearing a single hydroxymethyl substituent. It has a role as a solvent, a metabolite, an antioxidant and a fragrance.
製造方法
Benzyl alcohol is prepared commercially by the distillation of benzyl chloride with potassium or sodium carbonate. It may also be prepared by the Cannizzaro reaction of benzaldehyde and potassium hydroxide.
世界保健機関(WHO)
Benzyl alcohol has been used as an antimicrobial agent in
pharmaceutical preparations for many years. Parenteral administration of
preparations containing 0.9% benzyl alcohol resulted in the death of 16 neonates in
the USA in the early 1980s. Many countries subsequently warned against using
such preparations in neonates. This decision is not applicable to the use of benzyl
alcohol as a preservative in other circumstances or to its use in topical
preparations and no country has placed a total ban on the compound.
一般的な説明
A clear colorless liquid with a pleasant odor. Slightly denser than water. Flash point 194°F. Boiling point 401°F. Contact may irritate skin, eyes, and mucous membranes. May be slightly toxic by ingestion. Used to make other chemicals.
空気と水の反応
Slightly soluble in water.
反応プロフィール
Attacks plastics. [Handling Chemicals Safely 1980. p. 236]. Acetyl bromide reacts violently with alcohols or water [Merck 11th ed. 1989]. Mixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Example: an explosion will occur if dimethylbenzylcarbinol is added to 90% hydrogen peroxide then acidified with concentrated sulfuric acid. Mixtures of ethyl alcohol with concentrated hydrogen peroxide form powerful explosives. Mixtures of hydrogen peroxide and 1-phenyl-2-methyl propyl alcohol tend to explode if acidified with 70% sulfuric acid [Chem. Eng. News 45(43):73 1967; J, Org. Chem. 28:1893 1963]. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous-carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M 1991]. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence [Wischmeyer 1969].
危険性
Highly toxic.
健康ハザード
Benzyl alcohol is a low acute toxicant witha mild irritation effect on the skin. Theirritation in 24 hours from the pure compoundwas mild on rabbit skin and moderateon pig skin. A dose of 750 μg producedsevere eye irritation in rabbits. The toxicityof benzyl alcohol is of low order,the effects varying with the species. Oralintake of high concentrations of this compoundproduced behavioral effects in rats.The symptoms progressed from somnolenceand excitement to coma. Intravenous administrationin dogs produced ataxia, dyspnea,diarrhea, and hypermotility in the animals.
Adult and neonatal mice treated withbenzyl alcohol exhibited behavioral change,including sedation, dyspnea, and loss ofmotor function. Pretreatment with pyrazoleincreased the toxicity of benzyl alcohol. Withdisulfiram the toxicity remained unchanged.The study indicated that the acute toxicitywas due to the alcohol itself andnot to bezaldehyde, its primary metabolite(McCloskey et al. 1986).
火災危険
Benzyl alcohol is combustible.
応用例(製薬)
Benzyl alcohol is an antimicrobial preservative used in cosmetics,
foods, and a wide range of pharmaceutical formulations,
including oral and parenteral preparations, at concentrations up
to 2.0% v/v. The typical concentration used is 1% v/v, and it has
been reported to be used in protein, peptide and small molecule
products, although its frequency of use has fallen from 48 products
in 1996, 30 products in 2001, to 15 products in 2006. In
cosmetics, concentrations up to 3.0% v/v may be used as a
preservative. Concentrations of 5% v/v or more are employed as a
solubilizer, while a 10% v/v solution is used as a disinfectant.
Benzyl alcohol 10% v/v solutions also have some local anesthetic
properties, which are exploited in some parenterals, cough
products, ophthalmic solutions, ointments, and dermatological
aerosol sprays.
Although widely used as an antimicrobial preservative, benzyl
alcohol has been associated with some fatal adverse reactions when
administered to neonates. It is now recommended that parenteral
products preserved with benzyl alcohol, or other antimicrobial
preservatives, should not be used in newborn infants if at all
possible.
接触アレルゲン
Benzyl alcohol is mainly a preservative, mostly used in
topical antimycotic or corticosteroid ointments. It is
also a component catalyst for epoxy resins and is contained
in the color developer C-22. As a fragrance
allergen, it has to be mentioned by name in cosmetics
within the EU.
発がん性
In an NTP study, F344 rats were
dosed by oral gavage with 0, 200, and 400 mg/kg, 5 days/
week for 2 years. Benzyl alcohol had no effect on the survival
of male rats; female rats had reduced survival, and many of
the early deaths were considered related to the gavage
procedure. There were no treatment-related effects on nonneoplastic
or neoplastic lesions in either sex treated with
benzyl alcohol. It was concluded that under the conditions of
the study, there was no evidence of carcinogenic activity
. In the same NTP study, B6C3F1 mice were dosed by
oral gavage with 0, 100, and 200 mg/kg, 5 days/week for
2 years. No effects on survival or body weight gain were
observed. There were no treatment-related effects on nonneoplastic
or neoplastic lesions in either sex. It was concluded
that under the conditions of the study, there was no
evidence of carcinogenic activity.
環境運命予測
Biological. Heukelekian and Rand (1955) reported a 5-d BOD value of 1.55 g/g which is 61.5%
of the ThOD value of 2.52 g/g.
Chemical/Physical. Slowly oxidizes in air to benzaldehyde (Huntress and Mulliken, 1941).
Benzyl alcohol will not hydrolyze because it has no hydrolyzable functional group (Kollig, 1993).
代謝
Esters of benzyl alcohol are rapidly hydrolysed in vivo to benzyl alcohol, which is then oxidized . The animal organism readily oxidizes benzyl alcohol to benzoic acid, which after conjugation with glycine is rapidly eliminated as hippuric acid in the urine.
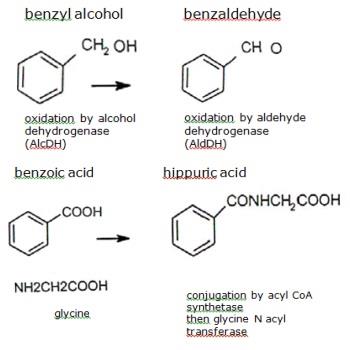
Benzyl alcohol is oxidised by alcohol dehydrogenase (AlcDH), a cytoplasmic enzyme present mainly in the liver, but also in the intestine and kidney. This reaction is saturable. The benzaldehyde formed is oxidised by aldehyde dehydrogenases (AldDH), cytoplasmic and mitochondrial enzymes mainly present in the liver, but also in the intestine and numerous organs.
貯蔵
Benzyl alcohol oxidizes slowly in air to benzaldehyde and benzoic
acid; it does not react with water. Aqueous solutions may be
sterilized by filtration or autoclaving; some solutions may generate
benzaldehyde during autoclaving.
Benzyl alcohol may be stored in metal or glass containers. Plastic
containers should not be used; exceptions to this include
polypropylene containers or vessels coated with inert fluorinated
polymers such as Teflon.
Benzyl alcohol should be stored in an airtight container,
protected from light, in a cool, dry place.
純化方法
It is usually purified by careful fractional distillation under reduced pressure in the absence of air. Benzaldehyde, if present, can be detected by UV absorption at 283nm. It has also been purified by shaking with aqueous KOH and extracting with peroxide-free diethyl ether. After washing with water, the extract is treated with saturated NaHS solution, filtered, washed, dried with CaO and distilled under reduced pressure [Mathews J Am Chem Soc 48 562 1926]. Peroxy compounds can be removed by shaking with a solution of Fe2+ followed by washing the alcohol layer with distilled water and fractionally distilling it. [Beilstein 6 IV 2222.]
不和合性
Benzyl alcohol is incompatible with oxidizing agents and strong
acids. It can also accelerate the autoxidation of fats.
Although antimicrobial activity is reduced in the presence of
nonionic surfactants, such as polysorbate 80, the reduction is less
than is the case with hydroxybenzoate esters or quaternary
ammonium compounds.
Benzyl alcohol is incompatible with methylcellulose and is only
slowly sorbed by closures composed of natural rubber, neoprene,
and butyl rubber closures, the resistance of which can be enhanced
by coating with fluorinated polymers. However, a 2% v/v
aqueous solution in a polyethylene container, stored at 208℃, may
lose up to 15% of its benzyl alcohol content in 13 weeks. Losses
to polyvinyl chloride and polypropylene containers under similar
conditions are usually negligible. Benzyl alcohol can damage
polystyrene syringes by extracting some soluble components
規制状況(Regulatory Status)
Included in the FDA Inactive Ingredients Database (dental
injections, oral capsules, solutions and tablets, topical, and vaginal
preparations). Included in parenteral and nonparenteral medicines
licensed in the UK. Included in the Canadian List of Acceptable
Non-medicinal Ingredients.
ベンジルアルコール 上流と下流の製品情報
原材料
準備製品