β-カロテン 化学特性,用途語,生産方法
外観
暗紫色~暗紫褐色, 結晶性粉末
定義
本品は、合成又は天然から得られるカロテノイド化合物であり、次の化学式で表される。
溶解性
クロロホルムにやや溶けやすく、シクロヘキサンに溶けにくく、エタノールに極めて溶けにくく、水にほとんど溶けない。
解説
カロチノイド色素の中で生物界に最も広く分布する代表的物質。カロテンとも呼ぶ。分子式C40H56を有する炭化水素。分子の中央に存在するポリエン鎖をはさむ両端のイオノン環の構造によりα,β,γ,δ,εなど多くの異性体が存在する。リコピンlycopeneもカロチンの異性体であり広義のカロチンに含むこともある。β‐カロチンはその中で最も広く分布しまたその量も多い。ニンジンの根,トウガラシの実,また緑葉中にクロロフィルとつねに共存する。
株式会社平凡社 世界大百科事典 第2版について 情報
用途
薬理研究用、黄色着色料。
用途
カロテン定量用標準品。
生体内での合成
さまざまな研究から、合成の原料としては酢酸が考えられており、これがメバロン酸を経て炭素原子5個(C5)のイソプレン単位ができ、さらに炭素原子がC10→C20→C40となり、異性化、段階的脱水反応による不飽和鎖の形成、ベンゼン環(C6)の形成を経てカロチンが生成すると考えられている。α-とβ-カロチンは緑葉中にかならず存在し、クロロフィルとともにみいだされ、光合成に関係していると考えられている。秋、クロロフィルが分解すると、それまで緑色で隠されていたカロチンの色が認められるようになる。しかし、鮮やかな赤や黄色の色素はカロチンでなく、アントシアンおよびタンニンの重合物である。4種のカロチンの分子式はいずれもC40H56で、互いに異性体である。
化粧品の成分用途
皮膚コンディショニング剤、着色剤
効能
紫外線保護剤
使用上の注意
不活性ガス封入空気及び光により変化し、発熱分解しやすい。最終的には、空気により自己発火することがある。
化学的特性
Yellow to orange solid.beta-Carotene is insoluble in water, but is available in water-dispersible, oil-dispersible and oil-soluble forms. It has the activity of vitamin A.
物理的性質
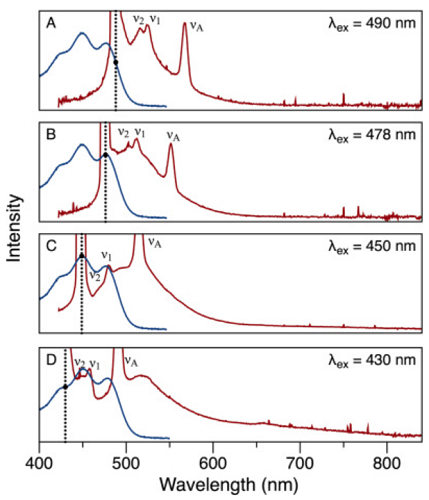
β-Carotene is a tetraterpene with 11 conjugated double bonds that give the molecule an orange color. It is a carotenoid compound that is present in large quantities in the human diet and subsequently is found in all human tissues, including blood. High temperature encourages the isomerization of the double bonds, which lightens the color. Absorption (blue) and fluorescence emission (red) spectra at four excitation wavelengths from β-carotene in hexane solvent at 23 °C are shown below.
天然物の起源
Beta-carotene is available naturally in fruits and vegetables. Synthetically, it may be manufactured from fungi or algae.
定義
ChEBI: A cyclic carotene obtained by dimerisation of all-trans-retinol. A strongly-coloured red-orange pigment abundant in plants and fruit and the most active and important provitamin A carotenoid.
一般的な説明
beta-Carotene is an antioxidant and is one of the most important carotenoids and a source of vitamin A. It is abundantly present in fruits and vegetables which is also used as a food supplement and a colorant.
安全性プロファイル
When heated to
decomposition it emits acrid smoke and
irritating fumes.
Source
The richest sources of β-Carotene are yellow, orange, and green leafy fruits and vegetables (such as carrots, spinach, lettuce, tomatoes, sweet potatoes, broccoli, cantaloupe, and winter squash). In general, the more intense the color of the fruit or vegetable, the more beta-carotene it has.

純化方法
It forms purple prisms when crystallised from *C6H6/MeOH and red rhombs from pet ether. Its solubility in hexane is 0.1% at 0o. It is oxygen sensitive and should be stored under N2 at -20o in the dark. It gives a deep blue colour with λmax at 590nm when mixed with SbCl3 in CHCl3. UV: (*C6H6) 429infl, max at 454 and 484nm. The principal peak at 454nm has 1cm 1% 2000. [Synthesis: Surmatis & Ofner J Org Chem 26 1171 1961; Milas et al. J Am Chem Soc 72 4844 1950.] β-Carotene is also purified by column chromatography (Al2O3 activity I-II). It is dissolved in pet ether/*C6H6 (10:1), applied to the column and eluted with pet ether/EtOH; the desired fraction is evaporated and the residue is recrystallised from *C6H6/MeOH (violet-red plates). [UV: Inhoffen et al. Justus Liebigs Ann Chem 570 54, 68 1950; Review: Fleming Selected Organic Synthesis (J Wiley, Lond) pp. 70-74 1973.] Alternatively it can be purified by chromatography on a magnesia column, thin layer of Kieselguhr or magnesia. Crystallise it from CS2/MeOH, Et2O/pet ether, acetone/pet ether or toluene/MeOH. Store it in the dark, under an inert atmosphere, at -20o. Recrystallise it also from 1:1 EtOH/CHCl3. [Bobrowski & Das J Phys Chem 89 5079 1985, Johnston & Scaiano J Am Chem Soc 1 0 8 2349 1986, Strain J Biol Chem 105 523 1934, Meth Biochem Anal 4 1 1957, Beilstein 5 II 638, 5 III 2453, 5 IV 2617.]
β-カロテン 上流と下流の製品情報
原材料
準備製品
β-カロテン-4,4'-ジオン
(2E,4E,6E,8E,10E,12E,14E,16E)-2,6,11,15-テトラメチル-17-(2,6,6-トリメチル-1-シクロヘキセニル)-2,4,6,8,10,12,14,16-ヘプタデカオクタエナール