イソブタン 化学特性,用途語,生産方法
種類
イソブタンは、主に工業用高圧ガスとして販売されています。ポリスチレン・ポリエチレンなどの発泡用に用いられる製品や、特殊なものでは、可燃・毒性ガス検知器校正用標準ガスなどがあります。
容器の種類は鋼製溶接容器などであり、所謂ガスボンベの形式で 販売されている物質です。内容量は50kg、500kgなどがあります。可燃・毒性ガス検知器校正用標準ガスは、可燃性ガスを使用する現場においてガス検知器の校正に使用されているものであり、空気で希釈されています。内容量は3.4L・10L・47Lなどです。
定義
本品は、次の化学式で表される炭化水素である。
性質
イソブタンは、分子量58.10、融点-159.42℃、沸点-11.7℃であり、常温での外観は無色気体です。
空気を1とした相対比重は2.01であり、密度は2.51kg/m3です。エタノール、エーテル、クロロホルムなどに溶解します。水への溶解度は48.9mg/Lです。
反応性
イソブタンは、強酸化剤、アセチレン、ハロゲンおよび窒素酸化物と反応して火災や爆発の危険を生じる物質です。また、空気より重い気体であるため、地面に沿って移動し、 流動、撹拌などにより、静電気が発生することがあります。そのため、遠距離発火の可能性が高いです。
解説
イソブタン,無色,特異臭をもつ気体.融点-159.60 ℃,沸点-11.73 ℃.容易に液化する.化学的には安定であるが,引火,爆発性がある.爆発範囲1.8~8.4体積%.液化石油ガスの成分として燃料に用いられるほか,オレフィンによりアルキル化されて,C6~C8 のイソパラフィン類(アルキレートガソリン)の製造原料,また脱水素によるイソブテン製造の原料に用いられる.
化粧品の成分用途
噴射剤、起泡剤
効能
噴霧剤
製造
イソブタン,脂肪族飽和炭化水素(アルカン)の一つ.分枝状パラフィンのもっとも簡単なもの.湿性天然ガス,石油系炭化水素の分解ガス中に含まれる.上記ガスより分離されるほかに,n-ブタンの異性化によっても生成される.構造は,プロパンの中心炭素と結合している水素の一つがメチル基と置換した形をとる.
危険性
イソブタンの主な危険性は、下記のものが挙げられます。
- 可燃性/引火性が高い
- 循環器系の障害
- 眠気またはめまいのおそれ
GHS分類では以下に分類されています。
- 可燃性/引火性ガス: 区分1
- 特定標的臓器毒性 (単回ばく露): 区分1 (循環器系) 、区分3 (麻酔作用)
化学的特性
2-Methylpropane (isobutane), C4H10, a flammable gas,
occurs in small quantities in natural gas and crude oil. It
has been detected in urban atmospheres at concentrations of
44–74 ppb. It also evolves from natural sources and
has been measured in diesel exhaust at 1.4–11 ppm
and in cigarette smoke at 10 ppm. The partition
coefficient of propane between olive oil and air at 37℃ is
12 using the method described by Sato and Nakajima and
Perbellini et al.. The lower explosive limit is
18,000 ppm in air.
使用
Isobutane occurs in petroleum, natural gas,and petroleum cracking products. It is usedas a fuel gas or a liquefied petroleum gas. Itis also used in organic synthesis.
定義
ChEBI: An alkane that is propane substituted by a methyl group at position 2.
一般的な説明
ISOBUTANE is a colorless gas with a faint petroleum-like odor. ISOBUTANE is shipped as a liquefied gas under its vapor pressure. Contact with the liquid can cause frostbite. ISOBUTANE is easily ignited. The vapors are heavier than air. Any leak can either be liquid or vapor. ISOBUTANE can asphyxiate by the displacement of air. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket.
空気と水の反応
Highly flammable.
反応プロフィール
ISOBUTANE is incompatible with the following: Strong oxidizers (e.g., nitrates & perchlorates), chlorine, fluorine, (nickel carbonyl + oxygen) .
危険性
Highly flammable, dangerous fire and
explosive risk; explosive limits in air 1.9–8.5%.
健康ハザード
Isobutane, like other saturated aliphatic alkanes,is nontoxic. It is an asphyxiate. Exposureto high concentrations of 1% in air maycause narcosis and drowsiness. Other thanthis, there is no report of any adverse healtheffect from exposure to this gas.
火災危険
EXTREMELY FLAMMABLE. Will be easily ignited by heat, sparks or flames. Will form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. CAUTION: Hydrogen (UN1049), Deuterium (UN1957), Hydrogen, refrigerated liquid (UN1966) and Methane (UN1971) are lighter than air and will rise. Hydrogen and Deuterium fires are difficult to detect since they burn with an invisible flame. Use an alternate method of detection (thermal camera, broom handle, etc.) Vapors may travel to source of ignition and flash back. Cylinders exposed to fire may vent and release flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
法規制情報
4. イソブタンの法規制情報
イソブタンは上記の危険性により、労働安全衛生法で、「名称等を表示し、又は通知すべき危険物及び有害物」「危険物・可燃性のガス」に指定されています。法令を遵守して正しく取り扱うことが重要です。
使用用途
イソブタンは、主にフロンガスの代替品として、エアコンや冷蔵庫の冷媒、スプレーなどに使用されています。アメリカ冷凍空調学会で定められた冷媒の種類を表す番号である冷媒番号は、R600aです。
また、イソブタンは、引火性及び爆発性があるため、液化ガスとして家庭用や工業用燃料に用いられている物質です。主にアウトドア仕様のカセットガスやライターに使われます。
沸点温度がブタンより低いため、寒冷地や冬山登山での使用に適性があります。また、その他の用途には有機合成原料、噴霧剤が挙げられ、イソブタンは特にイソパラフィン類の合成原料としても使用されている物質です。
化学反応
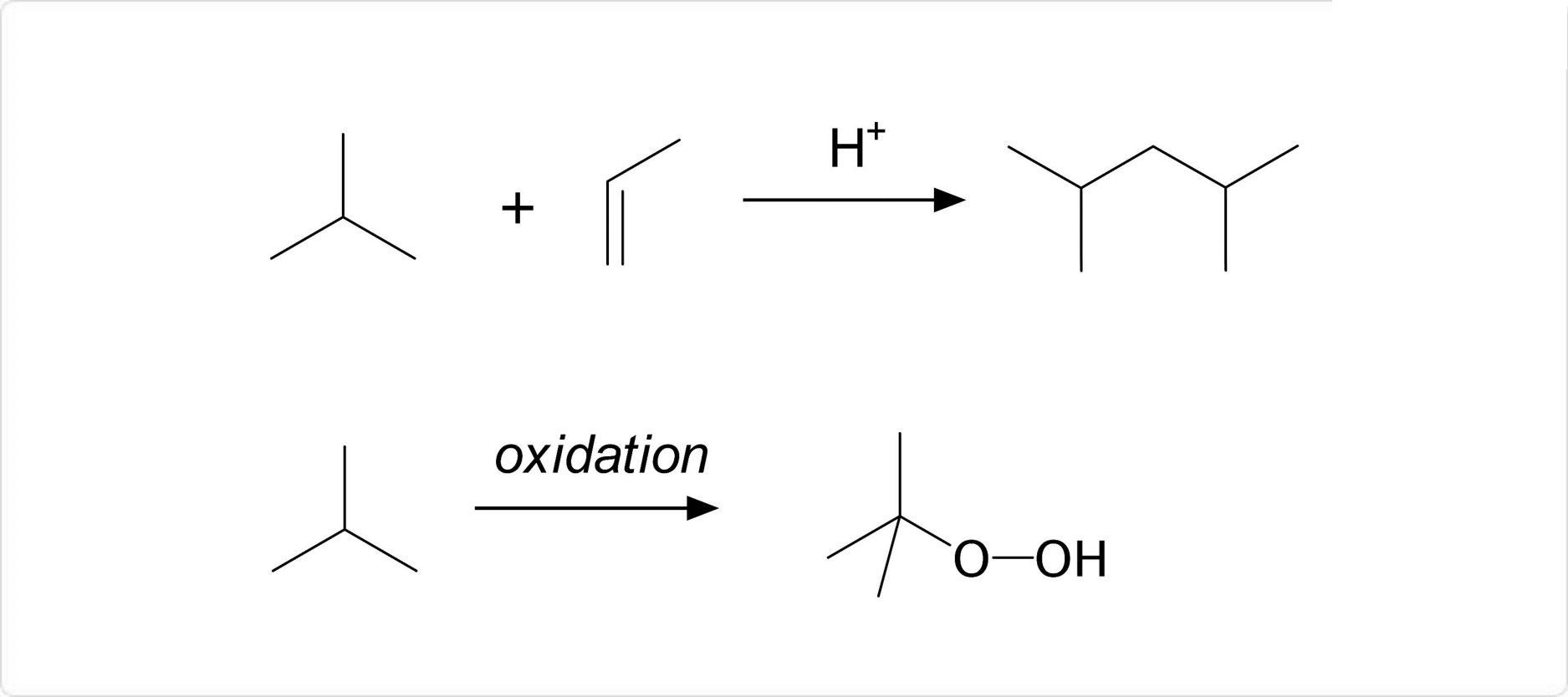
図1. イソブタンの化学反応
イソブタンは、酸性条件などで低分子アルケンと反応して炭化水素を生成します。この反応は、石油産業において2,4-ジメチルペンテンや2,2,4-トリメチルペンタンを合成するために用いられる反応です。
また、酸化によってtert-ブチルヒドロペルオキシドを生じます。この物質は、プロピレンと反応させることによりプロピレンオキサイドを合成することが可能です。
安全性プロファイル
An asphyxiant. A
common air contaminant. A very dangerous
fire and explosion hazard when exposed to
heat, flame, or oxidizers. To fight fire, stop
flow of gas. When heated to decomposition
it emits acrid smoke and irritating fumes.
環境運命予測
Photolytic. Based upon a photooxidation rate constant of 2.34 x 10
-12 cm
3/molecule?sec with OH
radicals in summer daylight, the atmospheric lifetime is 59 h (Altshuller, 1991). At atmospheric
pressure and 300 K, Darnall et al. (1978) reported a rate constant of 2.52 x 10
-12 cm
3/molecule?sec
for the same reaction. Rate constants of 1.28 x 10
-9 and 6.03 x 10
-12 L/molecule?sec were reported
for the reaction of 2-methylpropane with OH radicals in air at 300 and 296 K, respectively
(Greiner, 1967, 1970). Rate constants of 7.38 x 10
-13 and 6.50 x 10
-17 cm
3/molecule?sec were
reported for the reaction of 2-methylpropane with OH and NO3, respectively (Sablji? and Güsten,
1990).
Chemical/Physical. Complete combustion in air produces carbon dioxide and water vapor. 2-
Methylpropane will not hydrolyze because it does not contain a hydrolyzable functional group.
純化方法
Olefins and moisture can be removed by passage at 65o through a bed of silica-alumina catalyst which has previously been evacuated at about 400o. Alternatively, water and CO2 can be removed by passage through P2O5, then asbestos impregnated with NaOH. Treatment with anhydrous AlBr3 at 0o then removes traces of olefins. Inert gases can be separated by freezing the isobutane at -195o and evacuating out the system. [Beilstein 1 IV 282.]
イソブタン 上流と下流の製品情報
原材料
準備製品