1,2,3-ベンゾトリアゾール 化学特性,用途語,生産方法
外観
白色~わずかにうすい褐色, 結晶~結晶性粉末
溶解性
熱水に可溶, エタノール, アセトンに易溶。エタノール及びアセトンに溶けやすく、熱水に溶ける。
用途
紫外線吸収剤、写真薬(変色防止剤)
用途
有機合成原料。
用途
化学中間体、プラスチック安定剤、工業用水処理、写真防曇剤の腐食抑制剤、銅腐食防止剤
化学的特性
yellow to beige solid or Colorless needle-like crystals. Slightly soluble in cold water, ethanol and ether.
使用
1H-Benzotriazole is an anticorrosive agent, which is useful in aircraft deicing and antifreeze fluids. It is also employed in dishwasher detergents. Further, it is used as a restrainer in photographic emulsions and also useful as a reagent for the determination of silver in analytical chemistry. It also serves as a corrosion inhibitor in the atmosphere and underwater. Further, it is utilized in the synthesis of amines from glyoxal.
定義
ChEBI: 1H-Benzotriazole is the simplest member of the class of benzotriazoles that consists of a benzene nucleus fused to a 1H-1,2,3-triazole ring. It has a role as an environmental contaminant and a xenobiotic.
製造方法
1H-Benzotriazole is prepared by the reaction of o-phenylenediamine with nitrous acid in dilute sulfuric acid. Damschrodner and Peterson were able to synthesize the 1H-benzotriazole in a high yield (80%) by nitrosation of o-phenylenediamine with sodium nitrite in glacial acetic acid and water.
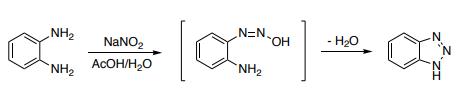
Synthesis of 1H-benzotriazole via diazotization of o-phenylenediamine
Reaction: Add o-phenylenediamine to 50°C water to dissolve, then add glacial acetic acid, cool down to 5°C, add sodium nitrite to stir the reaction. The reactant gradually turned dark green, the temperature rose to 70-80 ℃, the solution turned orange-red, placed at room temperature for 2 hours, cooled, filtered out the crystals, washed with ice water, dried to obtain the crude product, the crude product was distilled under reduced pressure, and collected 201 -204°C (2.0kPa) fraction, and then recrystallized with benzene to obtain 1H-Benzotriazole products with a melting point of 96-97°C, with a yield of about 80%.
主な応用
1H-Benzotriazole (BT) is a chemical used in a wide variety of industrial, commercial, and consumer products. It main used as an anticorrosive in metalworking, in art restoration, and as a tarnish remover and protective coating in the construction industry.
In the aircraft industry, 1H-benzotriazole and tolyl benzotriazole are found to be the primary agents in most types of aircraft deicing/antiicing fluid (ADAFs).
Benzotriazole is also used as a component of aircraft deicing fluid, pickling inhibitor in boiler scale removal, restrainer, developer and antifogging agent in photographic emulsions, corrosion inhibitor for copper, chemical intermediate for dyes, in pharmaceuticals, and as fungicide. (HSDB 1998).
Benzotriazole(BTA), ethylenediamine tetraaceticacid(EDTA), and potassium iodide(KI) were used for preparing the polishing slurries.
一般的な説明
White to light tan crystals or white powder. No odor.
空気と水の反応
Dust may form an explosive mixture in air. Slightly soluble in water.
反応プロフィール
The triazoles are a group of highly explosive materials that are sensitive to heat, friction, and impact. Sensitivity varies with the type substitution to the triazole ring. Metal chelated and halogen substitution of the triazol ring make for a particularly heat sensitive material. Azido and nitro derivatives have been employed as high explosives. No matter the derivative these materials should be treated as explosives.
危険性
Highly toxic by ingestion. May explode under vacuum distillation.
健康ハザード
ACUTE/CHRONIC HAZARDS: When heated to decomposition 1H-Benzotriazole emits toxic fumes. 1H-Benzotriazole can react violently during vacuum distillation.
火災危険
Flash point data are not available for 1H-Benzotriazole. 1H-Benzotriazole is probably combustible.
安全性プロファイル
Poison by intravenous route.Moderately toxic by ingestion and intraperitoneal routes.Questionable carcinogen with experimental tumorigenicdata. Mutation data reported. May detonate at 220°C or during vacuum distillation. When heated to decompositionit
職業ばく露
Because benzotriazoles are used in large quantities as a corrosion inhibitor, it is mainly through this type of use that benzotriazoles become an environmental contaminant. As a corrosion inhibitor and fire retardant, they are used in antifreeze in concentrations of 0.01-2.0% and in airplane deicing/antiicing fluids in unknown concentrations, up to 10% (Cancilla et al.,1997). Used antifreeze may leak or be poured down drains and thence enters the environment. Also, an estimated 80% of aircraft deicing/anti-icing fluids are deposited on the ground due to spray drift, jet blast, and wind shear during taxiing and takeoff, according to a recent study (Hartwell et al., 1995).
発がん性
Chronic (2-year) feeding studies
were conducted. Rats were given 0, 6700, or 12,000 ppm
in feed for 78 weeks and held for an additional 26 weeks.
Mice were given 0, 11,700, or 23,500 ppm in feed in
104 weeks. The authors concluded that under the conditions
shown in this study, there were no convincing
evidence that 1-H-benzotriazole was carcinogenic in rats
or mice.
純化方法
1,2,3-Benzotriazole crystallises from toluene, CHCl3, Me2NCHO or a saturated aqueous solution, and is dried at room temperature or in a vacuum oven at 65o. Losses are less if the material is distilled in a vacuum. CAUTION: may EXPLODE during distillation; necessary precautions must be taken. [Damschroder & Peterson Org Synth Coll Vol III 106 1955, Beilstein 26 III/IV 93.]
1,2,3-ベンゾトリアゾール 上流と下流の製品情報
原材料
準備製品