ホルマリン 化学特性,用途語,生産方法
外観
無色澄明の液体
定義
本品は、次の化学式で表される有機化合物である。
溶解性
水、エタノールに混和、ジエチルエーテルに不溶。水、エタノール及びアセトンに極めて溶けやすい。
解説
ホルムアルデヒド,メタナール(methanal)ともいう.有機物質の不完全燃焼ガス中や微量だが空気中にも存在する.実験室的には,メタノール蒸気と酸素を白金網上に通すと得られる.工業的には,メタノールの空気酸化法によって製造される.刺激性の無色の気体.融点-117 ℃,沸点-19.2 ℃.爆発範囲7~73体積%.水,メタノールなどの極性溶剤に易溶,無極性溶剤に難溶.非常に重合しやすいので,水溶液(ホルマリン)または重合状態(パラホルムアルデヒド)で利用される.おもな用途はフェノール樹脂,尿素樹脂,メラミン樹脂などの合成樹脂,およびイソプレン,ペンタエリトリトール,ヘキサメチレンテトラミンなどの製造原料,消毒用などに使われる.有毒.
用途
ポリアセタール樹脂?ユリア樹脂及びメラミン樹脂接着剤?フェノール樹脂?合成ゴム?メラミン樹脂(接着剤を除く)?ユリア樹脂(接着剤を除く)原料、溶剤、医薬?繊維処理剤?紙力増強剤?土木建築材料原料、キレート剤、農薬合成原料、石炭酸系?尿素系?メラミン系合成樹脂、農薬(失効農薬)、消毒剤
用途
タンパクを凝固させる作用があるため、消毒薬として医療器具(濃度1~5%)に用いられる。また、家具(濃度1~2%)や糊などの防腐剤、各種プレパラート作成の固定剤(濃度10~20%)としても用途が広い。2~3%に希釈したホルマリン溶液は、動物標本の作成にも用いられる。アンモニア性硝酸銀溶液にホルマリンを加えて温めると銀イオンを還元して銀を遊離する。これは「銀鏡反応」と呼ばれ、銀染色などに応用されている。その他有機合成原料、分析用等に極めて汎用される。
用途
病理組織固定剤。
用途
ホルマリンとは、ホルムアルデヒドの水溶液で、無色透明?刺激臭のある液体です。プラスチックスや樹脂、合成ゴム、塗料等の原料として使用されます。(別名:メチレンオキサイド?メタナール?メチルアルデヒド)
ホルマリンは、ポリアセタール樹脂(自動車、家電製品)、ユリア、メラミン樹脂接着剤(住宅)、フェノール樹脂(住宅、自動車、家電)、ペンタエリスリトール(塗料)、MDI(ウレタン原料)、パラホルムアルデヒド、合成ゴム、その他の原料として使用されます。
用途
病理組織の固定剤。
化粧品の成分用途
人工爪剤、爪コンディショニング剤
効能
殺菌消毒薬
特長
マイルドホルム10Nにメタノール10v/v%を添加しているため固定浸透性が優れている。
商品名
ホルマリン (健栄製薬); ホルマリン (大成薬品工業); ホルマリン (大成薬品工業); ホルマリン (小堺製薬); ホルマリン (小堺製薬); ホルマリン (小堺製薬); ホルマリン (小堺製薬); ホルマリン (山善製薬); ホルマリン (恵美須薬品化工); ホルマリン (東海製薬)
説明
Formaldehyde is a colorless, flammable gas with a distinctive pungent odor. It is the simplest
aldehyde, which is a class of organic compounds with the carbonyl group bonded to at
least one hydrogen atom. Formaldehyde was described by August Wilhelm von Hoff mann
(1818–1892) in 1867 after the Russian Aleksandr Butlerov (1828–1886) had inadvertently
synthesized it in 1857. Formaldehyde readily dissolves in water to produce a solution called
formalin, which is commonly marketed as a 37% solution.
化学的特性
Formaldehyde is an important chemical widely used by industry to manufacture building materials and numerous household products. It is also a by-product of combustion and certain other natural processes. It is present in substantial concentrations both indoors and outdoors. Formaldehyde is well known as a preservative in medical laboratories, as an embalming fl uid, and as a sterilizer. Its primary use is in the production of resins and as a chemical intermediate. Urea formaldehyde (uf) and phenol formaldehyde (pf) resins are used in foam insulations, as adhesives in the production of particle board and plywood, and in the treating of textiles. Sources of formaldehyde in the home include building materials, smoking, household products, and the use of unvented, fuel-burning appliances, like gas stoves or kerosene space heaters. Formaldehyde, by itself or in combination with other chemicals, serves a number of purposes in manufactured products. It has been reported that the use and production of formaldehyde in 1998 was about 11.3 billion pounds and the international production crossed over 46 billion pounds in 2004.
物理的性質
Formaldehyde is a clear, colorless liquid with a pungent, suffocating odor. Burning taste. Experimentally determined odor threshold concentrations of 1.0 ppmv and 0.50 ppmv were reported by Leonardos et al. (1969) and Nagata and Takeuchi (1990), respectively. Also,formalin is an aqueous solution that is 37% formaldehyde by weight; inhibited solutions (added to prevent polymeri zation) usually contain 6 12% methyl alcohol. Formaldehyde is used in the manufacture of plastics and resins by reaction with phenols,urea, and melamine. It is used as a preservative,a disinfectant, and as a chemical intermediate.
来歴
Formaldehyde is a by-product of combustion of organic compounds, metabolism, and
other natural processes. Formaldehyde results from wood combustion and elevated atmospheric
concentrations can result from forest fires, as well as from urban pollution sources
such as transportation. Formaldehyde has been identified as a significant indoor air pollutant.
Building materials such as particleboard, plywood, and paneling are major sources of formaldehyde
because they incorporate formaldehyde resins as bonding adhesives. Other sources of
formaldehyde in the home are carpets, upholstery, drapes, tobacco smoke, and indoor combustion
products. Formaldehyde may be emitted from building materials for several years after
installation. In the two decades of the 1960s and 1970s, a half million homes in the United
States used urea formaldehyde foam insulation, but health complaints led to its elimination
as an insulator in the early 1980s. People react differently to formaldehyde exposure, but it is
estimated that between 10% and 20% of the population will experience some reaction at concentrations
as low as 0.2 parts per million. Formaldehyde irritates the eyes, nose, and throats,
producing coughing, sneezing, runny nose, and burning eyes. More severe reactions result in insomnia, headaches, rashes, and breathing difficulties. Some states have established indoor air
quality standards ranging from 0.05 to 0.5 ppm.
使用
Formaldehyde is used in the manufactureof phenolic resins, cellulose esters, artificialsilk, dyes, explosives, and organic chemicals.Other uses are as a germicide, fungicide, anddisinfectant; in tanning, adhesives, waterproofingfabrics, and for tonic and chromeprinting in photography; and for treating skindiseases in animals. In vitro neutralizationof scorpion venom toxicity by formaldehydehas been reported (Venkateswarlu et al.1988).
Formaldehyde constitutes about 50% ofall aldehydes present in the air. It is oneof the toxic effluent gases emitted fromburning wood and synthetic polymeric substancessuch as polyethylene, nylon 6, andpolyurethane foams. Firefighters have a greaterrisk to its exposure. Incapacitation fromthe toxic effluent gases is reported to occurmore rapidly from the combustion of syntheticpolymers than from that of naturalcellulose materials.
Formaldehyde is directly emitted into theair from vehicles. It is released in traceamounts from pressed wood products suchas particleboard and plywood paneling, fromold “sick” buildings, and from cotton andcotton–polyester fabrics with selected crosslinkfinishes. Formation of formaldehyde hasbeen observed in some frozen gadoid fishdue to enzymic decomposition of the additivetrimethylamine oxide (Rehbein 1985).Its concentration can build up during frozenstorage of fish (Leblanc and Leblanc 1988;Reece 1985). It occurs in the upper atmosphere,cloud, and fog; it also forms inphotochemical smog processes.
定義
Formalin: a colourless solution of methanal (formaldehyde) in waterwith methanol as a stabilizer; r.d.1.075–1.085. When kept at temperaturesbelow 25°C a white polymer ofmethanal separates out. It is used asa disinfectant and preservative forbiological specimens.
調製方法
The industrial preparation of formaldehyde has occurred since the late 1800s and involvesthe catalytic oxidation of methanol: 2CH
3OH(g) + O
2(g) → 2CH
2O(g).the oxidationtakes place at temperatures between 400°C and 700°C in the presence of metal catalysts. Metalsinclude silver, copper, molybdenum, platinum, and alloys of these metals. Formaldehyde iscommonly used as an aqueous solution called formalin. Commercial formalin solutions varybetween 37% and 50% formaldehyde. When formalin is prepared, it must be heated anda methanol must be added to prevent polymerization; the final formalin solution containsbetween 5% and 15% alcohol.
製造方法
Formalin is adjusted to pH 8 and urea is added to give a urea to
formaldehyde ratio of about 1 :2.5 molar. The resulting solution is boiled
under reflux for 1 hour. Butanol (1.5-2.0 mole per mole of urea) is then added
together with a little xylene. The latter forms, with butanol and water, a
ternary azeotrope which on distillation yields a condensate separating into an
upper organic layer and a lower aqueous layer. By discarding the lower layer
and returning the upper layer to the reactor, water is progressively removed
from the system. After a substantial proportion of the water has been
removed, an acid catalyst (e.g. phosphoric acid or phthalic anhydride) is
added and heating is continued. When the required degree of reaction is
attained, the solution is neutralized and concentrated to the desired solids
content.
毒性
ホルムアルデヒドは生活環境中では建材、塗料、接着剤などの原料として使われているが、毒性が強く、視覚障害、皮膚や消化器の炎症、鼻や咽頭(いんとう)の粘膜の刺激などの症状を引き起こす。木材、石炭、砂糖など多くの有機化合物が不完全燃焼する際の煙にはホルムアルデヒドが含まれているので、目や鼻の粘膜が刺激され、痛みや炎症をおこす。また、建材や建築用接着剤に含まれているホルムアルデヒドはシックハウス症候群
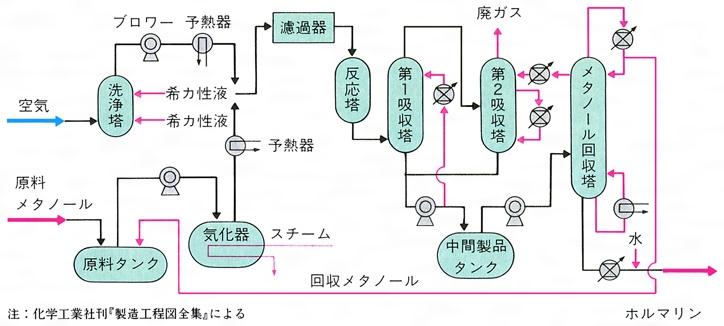
工業的製法
メタノール(メチルアルコール)の接触酸化によって生成する。この反応は、メタノールの蒸気と酸素を赤熱した白金、銅、または銀網上に通すとおこり、反応熱が触媒を熱するから触媒を加熱し続ける必要がない。工業的にも、メタノールと空気の混合ガスを加熱して銀またはモリブデン鉄混合酸化物触媒上に通して反応させると得られる。この反応はメタノール‐空気混合ガスの爆発範囲(メタノール7~37%)を避けて行う必要があり、そのためにメタノールを約50%含む混合ガスを用いるメタノール過剰法と、メタノールを約5%にした空気過剰法の2通りの製法がある。現在では、空気の使用量が少なく、動力の点で有利な前者がおもな工業的製法になっている。
一般的な説明
Formaldehyde solution commercially formaldehyde solution is an aqueous solution containing 37% formaldehyde and 8-10% methanol. Formaldehyde solution can be activated by adding the catalytic amount of lanthanide triflate. This activated formaldehyde solution was employed for the smooth hydroxymethylation reaction of silyl enol ethers. Formaldehyde solution reacts with silyl enol ethers to afford the corresponding hydroxymethylated adducts in high yields.
空気と水の反応
The solution gives up formaldehyde vapors readily. These vapors are flammable over a wide vapor-air concentration range. Water soluble.
反応プロフィール
FORMALDEHYDE, SOLUTION, reacts violently with strong oxidizing agents (hydrogen peroxide, performic acid, perchloric acid in the presence of aniline, potassium permanganate, nitromethane). Reacts with bases (sodium hydroxide, potassium hydroxide, ammonia), and with nitrogen dioxide (explosive reaction around 180°C). Reacts with hydrochloric acid to form highly toxic bis(chloromethyl) ether. Polymerization reaction with phenol may develop sudden destructive pressure [Bretherick, 5th ed., 1995, p.168].
危険性
Moderate fire risk. Explosive limits in air 7–
73%. Toxic by inhalation, strong irritant, a carcinogen. (Solution) Avoid breathing vapor and avoid
skin contact. Confirmed carcinogen.
健康ハザード
Formaldehyde is moderately toxic by skin contact and inhalation. Exposure to
formaldehyde gas can cause irritation of the eyes and respiratory tract, coughing, dry
throat, tightening of the chest, headache, a sensation of pressure in the head, and
palpitations of the heart. Exposure to 0.1 to 5 ppm causes irritation of the eyes, nose, and
throat; above 10 ppm severe lacrimation occurs, burning in the nose and throat is
experienced, and breathing becomes difficult. Acute exposure to concentrations above 25
ppm can cause serious injury, including fatal pulmonary edema. Formaldehyde has low
acute toxicity via the oral route. Ingestion can cause irritation of the mouth, throat, and
stomach, nausea, vomiting, convulsions, and coma. An oral dose of 30 to 100 mL of 37%
formalin can be fatal in humans. Formalin solutions can cause severe eye burns and loss
of vision. Eye contact may lead to delayed effects that are not appreciably eased by eye
washing.Formaldehyde is regulated by OSHA as a carcinogen (Standard 1910.1048) and is
listed in IARC Group 2A ("probable human carcinogen"). This substance is
classified as a "select carcinogen" under the criteria of the OSHA Laboratory
Standard. Prolonged or repeated exposure to formaldehyde can cause dermatitis and
sensitization of the skin and respiratory tract. Following skin contact, a symptom free period may occur in sensitized individuals. Subsequent exposures can then lead
to itching, redness, and the formation of blisters
燃焼性と爆発性
Formaldehyde gas is extremely flammable; formalin solution is a combustible liquid (NFPA rating = 2 for 37% formaldehyde (15% methanol), NFPA rating = 4 for 37% formaldehyde (methanol free)). Toxic vapors may be given off in a fire. Carbon dioxide or dry chemical extinguishers should be used to fight formaldehyde fires.
化学反応性
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
农业用途
Microbiocide, Fungicide, Bactericide; Soil
sterilent: Registered for use in the U.S. Not approved for
use in EU countries. Formaldehyde has found wide
industrial usage as a fungicide, germicide and in disinfectants. It is used most often in an aqueous solution
stabilized with methanol (formalin). It is also a pesticide
intermediate.
製品名
DYNOFORM®; FANNOFORM®;
FORMALITH®; FORMOL®; FYDE®; HERCULES
37 M6-8®; HOCH®; IVALON®; KARSAN®;
LYSOFORM®; MAGNIFLOC 156C FLOCCULANT®;
MORBICID®; STERIFORM®; SUPERLYSOFORM®
接触アレルゲン
Sources and uses of formaldehyde are numerous. Exposed
people are mainly health workers, cleaners, painters, met alworkers, but also photographers (color developers) and
carbonless copy paper users. Formaldehyde can induce
contact urticaria. Formaldehyde may be the cause of sen sitization to formaldehyde releasers: benzylhemiformal,
bromonitrodioxane, bromonitropropanediol (?), chloroal lylhexaminium chloride or Quaternium-15, diazolidinylu rea, dimethylol urea, dimethyloldimethylhydantoin or
DMDM hydantoin, hexamethylenetetramine or methe namine, imidazolidinylurea, monomethyloldimethylhy dantoin or MDM hydantoin, N-methylolchloracetamide,
paraformaldehyde and trihydroxyethylhexahydrotriazine
or Grotan BK.
Formaldehyde is used for the synthesis of many resins.
Some of them, such as formaldehyde-urea and melamine formaldehyde resins, can be used in textiles and second arily release free formaldehyde (see Chap. 40).
Other resins, such as p-tert-butylphenol formalde hyde resin or tosylamine formaldehyde resin, do not
release formaldehyde.
職業ばく露
Formaldehyde has found wide indus trial usage as a fungicide, germicide; and in disinfectants
and embalming fluids. It is also used in the manufacture of
artificial silk and textiles, latex, phenol, urea, thiourea and
melamine resins; dyes, and inks; cellulose esters and other
organic molecules; mirrors, and explosives. It is also used
in the paper, photographic, and furniture industries. It is an
intermediate in drug manufacture and is a pesticide
intermediate.
発がん性
Formaldehyde is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans and supporting data on mechanisms of carcinogenesis. Formaldehyde was first listed in the Second Annual Report on Carcinogens in 1981 as reasonably anticipated to be a human carcinogen based on sufficient evidence from studies in experimental animals. Since that time, additional cancer studies in humans have been published, and the listing status was changed to known to be a human carcinogen in the Twelfth Report on Carcinogens (2011).
環境運命予測
Biological. Biodegradation products reported include formic acid and ethanol, each of which can further degrade to carbon dioxide (Verschueren, 1983).
Photolytic. Major products reported from the photooxidation of formaldehyde with nitrogen oxides are carbon monoxide, carbon dioxide and hydrogen peroxide (Altshuller, 1983). In synthetic air, photolysis of formaldehyde gave hydrochloric acid and
Irradiation of gaseous formaldehyde containing an excess of nitrogen dioxide over chlorine yielded ozone, carbon monoxide, nitrogen pentoxide, nitryl chloride, nitric acid and hydrochloric acid. Peroxynitric acid was the major photolysis product when chlo
Chemical/Physical. Oxidizes in air to formic acid (Hartley and Kidd, 1987). Trioxymethylene may precipitate under cold temperatures (Sax, 1984). Polymerizes easily (Windholz et al., 1983). Anticipated products from the reaction of formaldehyde with ozone orhydroxyl radicals in air are carbon monoxide and carbon dioxide (Cupitt, 1980). Major products reported from the photooxidation of formaldehyde with nitrogen oxides are carbon monoxide, carbon dioxide and hydrogen peroxide (Altshuller, 1983).
Reacts with hydrochloric acid in moist air forming bis(chloromethyl)ether. This compound may also form from an acidic solution containing chloride ion and formaldehyde (Frankel et al., 1974). In an aqueous solution at 25°C, nearly all the formaldehyde add
貯蔵
work with formaldehyde should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Formaldehyde should be used only in areas free of ignition sources. Containers of formaldehyde should be stored in secondary containers in areas separate from oxidizers and bases.
輸送方法
UN1198 Formaldehyde solutions, flammable,
Hazard Class: 3; Labels: 3-Flammable liquid, 8-Corrosive
material. Cylinders must be transported in a secure upright
position, in a well-ventilated truck. Protect cylinder and
labels from physical damage. The owner of the compressed
gas cylinder is the only entity allowed by federal law
(49CFR) to transport and refill them. It is a violation of
transportation regulations to refill compressed gas cylinders
without the express written permission of the owner.
UN2209 Formaldehyde solutions, with not<25% formal dehyde, Hazard class: 8; Labels: 8-Corrosive material.
UN3077 For solids containing varying amounts of formal dehyde : UN3077
Environmentally hazardous substances, solid, n.o.s., Hazard
class: 9; Labels: 9-Miscellaneous hazardous material,
Technical Name Required.
純化方法
It commonly contains added MeOH. Add KOH solution (1 mole KOH: 100 moles HCHO) to ~37% by weight aqueous formaldehyde solution (formalin), or evaporate to dryness, to give paraformaldehyde polymer which, after washing with water, is dried in a vacuum desiccator over P2O5 or H2SO4. Formaldehyde is regenerated by heating the paraformaldehyde to 120o under vacuum, or by decomposing it with barium peroxide. The monomer, a colourless flammable gas, is passed through a glass-wool filter cooled to -48o in a CaCl2/ice mixture to remove particles of polymer, then dried by passage over P2O5 and either condensed in a bulb immersed in liquid nitrogen or absorbed in ice-cold conductivity water. The gas or aqueous solutions have pungent suffocating odours, are LACHRYMATORY and suspected carcinogens, handle carefully. Formalin is a disinfectant and a preservative of dead animal and plant tissues. [Beilstein 1 IV 3017.]
不和合性
Pure formaldehyde may polymerize
unless properly inhibited (usually with methanol). May
form explosive mixture with air. Incompatible with strong
acids; amines, strong oxidizers; alkaline materials; nitrogen
dioxide; performic acid; phenols, urea. Reaction with
hydrochloric acid forms bis-chloromethyl ether, a carcino gen. Formalin is incompatible with strong oxidizers, alkalis,
acids, phenols, urea, oxides, isocyanates, caustics,
anhydrides.
廃棄物の処理
Return refillable compressed
gas cylinders to supplier. Incineration in solution of combus tible solvent. Consult with environmental regulatory agen cies for guidance on acceptable disposal practices. Generators
of waste containing this contaminant (≥100 kg/mo)
must conform with EPA regulations governing storage, trans portation, treatment, and waste disposal.
ホルマリン 上流と下流の製品情報
原材料
準備製品
Early-strength admixture
N-エチル-N-[4-[[4-[N-エチル-N-(3-ソジオスルホベンジル)アミノ]フェニル][4-(ジエチルアミノ)-2-メチルフェニル]メチレン]-2,5-シクロヘキサジエン-1-イリデン]-3-スルホナトベンゼンメタンアミニウム
CMC gum
ureaformaldelyde resin UF
cis-2-ブテン-1,4-ジオール
N-ヒドロキシメチルフタルイミド
オレイン酸カリウム
4,4'-メチレンビス(2-クロロアニリン)
ベンジルクロロメチルエーテル
Dye-fixing agent M
Dicyanide diamino formaldehyde,condensation compound
butadiene-styrene-pyridine copolymer rubber latex
Dye-fixing agent G
Ethyl α-cyanoacrylate instantaneous adhesive
りん酸ほう素
dimethyl dodecyl thioic propylene betaine
Diffusing agent NNO
ジエチレントリアミン五酢酸
イソシアン酸
Octadecylamine N-oleoyl Sarcosinate
Dispersing agent DN
5-(ヒドロキシメチル)ウラシル
塩化4-メトキシ-3-メチルベンジル
ヒドロキシメタンスルホン酸ナトリウム
4tertブチルフェノル·ホルムアルデヒド重
Modified glutaraldehyde
Amino moulding plastic
1-(2-ヒドロキシエチル)-4-メチルピペラジン
N-フェニルグリシン
3-ヒドロキシ-6-メチル-2-ピリジンメタノール
2,6-ヘプタンジオン
2,2'-メチレンビス(6-tert-ブチル-p-クレゾール)
Adhesive 706
softener MS
ポリ(オキシメチレン)
1-METHYL-PIPERIDINE-4-CARBONITRILE
2-ヒドロキシベンジルアルコール
N-メチルプロピルアミン
L-1,2,3,4-TETRAHYDROISOQUINOLINE-3-CARBOXYLIC ACID HYDROCHLORIDE, 97
color fixing agent Y